|
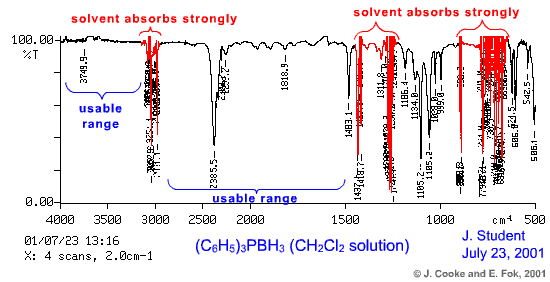
Preparing a Solution IR Sample Samples that are soluble in suitable organic solvents may be recorded in solution. Special sealed cells are used for this application. The two KCl IR plates are separated by a spacer (0.1 - 0.5 mm thickness) and the solution is admitted to the cell by the use of two stainless steel ports. Because the sample is prepared as a solution, you must first obtain a background spectrum of the solvent itself. The solvent background will then be subtracted from that of the (solvent+sample) spectrum. The fact that the KCl plates are held a fixed width apart means that the solvent background can be obtained precisely (in contrast, you cannot subtract Nujol from a Nujol mull because the thickness of the film varies). Because the solvent will absorb in certain regions of the IR spectrum, it is important to choose a solvent that does not absorb in the region of interest. CHCl3 and CH2Cl2 are reasonably transparent to infrared radiation in the region 2800 - 1600 cm-1. If you have selected the solvent wisely, you should find that the baseline is quite flat and very near to 100% T.
A Few Practical Problems to Watch For
|
||||||||
|
Contact the Webmaster |
||||||||