| |
Back | Contents | Next
Preparing a Cast Film IR Sample
The cast film is similar in scope to the thin film method with two exceptions - only one salt plate is used and the solid must first be soluble in a low boiling, anhydrous organic solvent to introduce it onto the salt plate. The cast film has the advantage that the recorded IR spectrum is exclusively of the sample compound, but has the disadvantage that there are a number of practical problems in obtaining good quality spectra.
- Dissolve a few mg of the solid compound in several drops of an appropriate low-boiling solvent (e.g., CH2Cl2 or CHCl3).
- Place one or two drops of the solution in the center of the IR plate with a disposable pipet.
- Let the solvent evaporate.
- it is important that the solvent be allowed to evaporate completely; otherwise, solvent bands might also appear in the IR spectum
- Rest the single IR plate in the sample mount and gently press and twist the cover on top.
- Record the background.
- Record the spectrum.
- Clean up.
A Few Practical Problems to Watch For
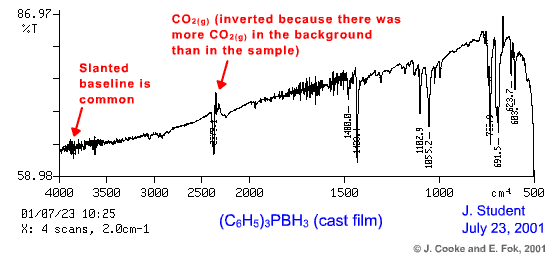
Click here to see the corresponding sample recorded as a Nujol mull
- if the distortion is mild, you may be able to correct the baseline
- if too much solid has been deposited, the radiation may not be able to pass through and reach the detector, resulting in very low %Transmittance values and poor spectral quality.
- good spectra are generally obtained when the deposited solid resembles a transparent film
- conversely, poor spectra often result when the film is opaque

A good cast film
|

A poor cast film
|
- some trial and error will be required to attain the correct conditions
- you will find that you will have to strike a balance between applying enough solid to observe a spectrum of your product while at the same time keeping the film thin enough so that the IR beam can reach the detector unimpeded
Top
Back | Contents | Next
|
|