| |
Back | Contents | Next
Troubleshooting
(Note: the two problems discussed on this page often (but not always) occur simultaneously)
Problem: My spectrum has a slanted baseline.
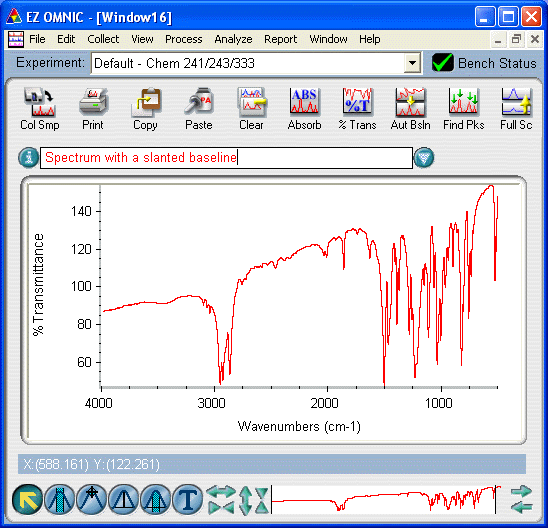
- the incident IR beam is scattering rather than passing through the sample. Most often, the baseline will slant so that high wavenumbers have low %T values and low wavenumbers will have high %T values. The causes for this include
- if you prepared a cast film of a solid sample (such as the above example), this is a normal effect. If the baseline is not too badly slanted, you may be able to correct the baseline. If not, you will have to prepare a new cast film and try again.
- if you prepared a Nujol mull of a solid sample, this likely means that the particle size is too large. If the baseline is not too badly slanted, you may be able to correct the baseline. If not, you will have to prepare a new Nujol mull and try again, this time being sure to finely grind the powder.
- if the IR plate is badly scratched or cloudy, it will sometimes result in a slanted baseline (for all sample types except the solution method). If you suspect this is the case, contact your T.A. or the Laboratory Coordinator.
- if you prepared a KBr disk of a solid sample, this likely means that your disk was not "glassy" enough and the remaining particles of powdered KBr are scattering the beam. If the baseline is not too badly slanted, you may be able to correct the baseline. If not, you will have to prepare a new KBr disk and try again, this time applying more pressure and/or leaving the pellet under high pressure for a longer period of time.

Problem: The baseline of my spectrum does not fall near 100%T.
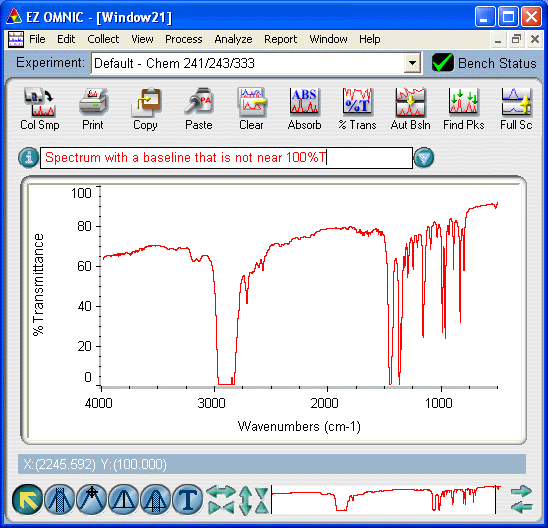
- your sample is absorbing IR radiation in areas to which it should theoretically be transparent (i.e., areas where the IR beam should pass unimpeeded, registering a signal at 100%T). In practice, a baseline within about 80-120%T is acceptable, except for solution samples where the baseline should fall very near the ideal value. The causes for this include
- if you prepared a KBr disk of a solid sample, this is a normal effect; while glassy KBr is theoretically transparent to IR radiation, this is difficult to achieve in practice. If the baseline is not too badly slanted, you may be able to correct the baseline. If not, you will have to prepare a new KBr disk and try again.
- for a thin film, this effect is rare, but will happen if the sample is too thick. In this case, you will also find that your spectrum is too strong (peaks have flat tops). Try reducing the amount of liquid you put on the plate or removing some of the existing liquid with a Kimwipe.
- for a cast film, the deposited solid is too thick. You will often also find that your spectrum has a slanted baseline. Try diluting your solution and preparing the cast again.
- for a Nujol mull, it is likely that there is too much mull between the IR plates. Try separating the plates, wiping one clean with a KimWipe, reforming the sandwich of plates and recording the sample spectrum again.
- for a solution, it is most likely that the solvent background was not subtracted properly. If this is the case, there are likely also spurious signals from the solvent in the spectrum. Try recording the background and sample spectra again.
- this effect would be observed if you removed the cell from the sample compartment before either the background or sample spectrum had finished scanning
- if the IR plate is badly scratched or cloudy, it will sometimes result in poor transmission (for all sample types except the solution method). If you suspect this is the case, contact your T.A. or the Laboratory Coordinator.

Top
Back | Contents | Next
|
|