For a long-time, we have been interested in the potential of photochemical reactions for accomplishing otherwise elusive synthetic transformations. An area of particular focus has been the photochemical crossed [4+4]-cycloaddition of pyran-2-ones. This process joins together two remote 4p systems in a photochemically allowed 4+4 construction, resulting in the direct formation of a new cyclooctadiene bridged by an ether and a lactone ring
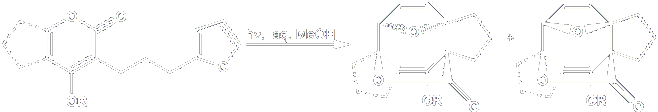
Given the inherent challenge in constructing cyclooctanoid systems, a reaction that directly forms a highly functionalized 8-membered ring fused to a second ring can clearly function as a strategy-level transformation for the rapid assembly of cyclooctane-containing targets. Our efforts to exploit this methodology in the total syntheses of the diterpene natural products Traversianal and are brefeily described below.
|




