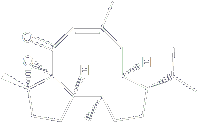
As noted, the crossed [4+4]-photocycloaddition of pyran-2-ones offers an attractive entry to cyclooctanoid systems. This methodology are currently being used towards the synthesis of the two natural product Traversianal and 7 ,8-Epoxy-4-basmen-6-one. |
|
This cembranoid natural product was isolated in 1983 from Greek tobacco (Serres). is a carbotricyclic diterpene with an epoxy group as its most challenging functional group.
|
In efforts to exploit our carben/ylide methodology, three different natural products are currently under
investigation; Dactylol, Laurencin and Phorbol. By using small heteroatom-containing ring systems as starting material, the desired medium sized rings can be obtained through a stereoselective 1,2-yide shift.
|
|
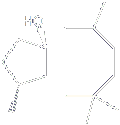
With its fused 5-8 ring system, Dactylol is an irregular isoprenoid sesquiterpene alcohol that was isolated in 1978 from the Caribbean sea hare Aplysia dactylomela. It has later also been found in the red seaweed Laurencia poitei.
|
|
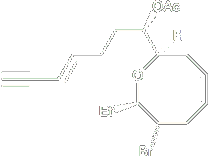
This marine natural product, a halogenated C 15 acetogenin, consisting of a challenging oxocene core, was first isolated from the red alga Laurencia glandulifera in 1965. With its 8-membered ring and functionality pattern the molecule pose a significant synthetic challenge.
|
|
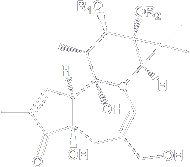
This tigliane diterpene with an interesting 5-7-6-3 fused ring system was isolated from the seed oil Croton tiglium in 1967. Phorbol esters have long been implicated in the progression of carcinogenesis following initial mutation events, apparently through their ability to stimulate the protein kinase C (PKC) signal transduction pathway in transformed cells.
|
|




