Several members of our group are currently investigating the Nazarov reaction, a 4p-electrocyclization forming cyclopentenones. The ability of the Nazarov intermediates to undergo either inter- or intramolecular trapping with various nucleophiles has enabled the formation of complex ring structures.
The oxy-allyl cation formed through the Nazarov cyclization has been shown to undergo intermolecular [4+3] cycloadditions with various dienes to afford formal [4+4] products.
|
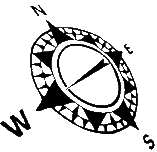
Certain classes of electron-rich alkenes can trap the Nazarov intermediate in a stepwise 3+2 annulation process to give bicyclo[2.2.1] heptanones. Readily available vinyl sulfides are especially effective in this process, and the sulfur group in the resulting adduct provides a convenient handle for further elaboration.
|
|
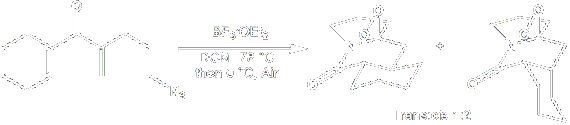
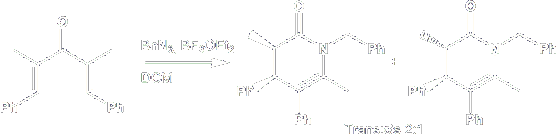
Mono- and bicyclic 1-aza-2-oxo ring systems were synthesized by the inter- and intramolecular trapping of the Nazarov oxyallyl intermediate with azides.
|
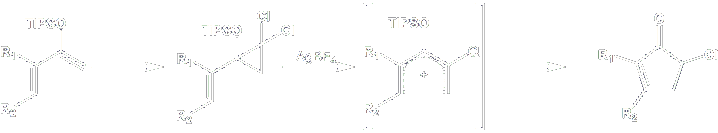
Dihalocyclopropanes are easily prepared via carbene-based methods. Further ionized with concomitant electrocyclic opening to allyl cations. We have recently proved this process to initiate a a novel
method for carrying out the Nazarov reaction.
|
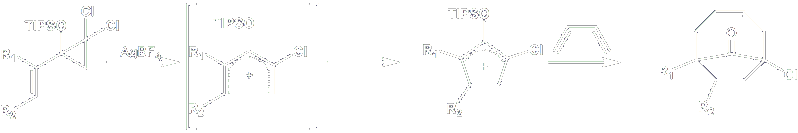
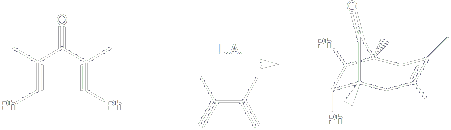
To further explore this new approach to the Nazarov reaction, we are currently seeking to trap the oxy-allyl cation intermediate using different dienes. As above, we wish to accomplish the [4+3] cycloadditions to afford formal [4+4] products.
|
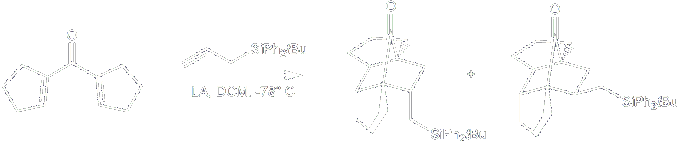
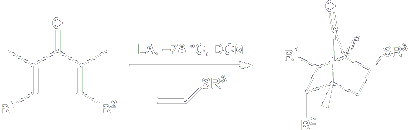
By using allyl-silanes as traps we can construct a variety of [2,1,2]-bicyclic heptanones with a pendant protected silyl group. Oxidation of the silyl group using Flemming-Tamao conditions, can give a convenient hydroxyl group for further elaboration.
|

A complementary reaction to the Nazarov reaction would be the anionic Nazarov, or cyclization of a pentadienyl anion. This anion can be derived by attack of a vinyl allene by an anion-stabilizing nucleophile. This should, in theory, cyclize through a disrotatory ring closure, giving the cyclopentene. Studies are underway
|
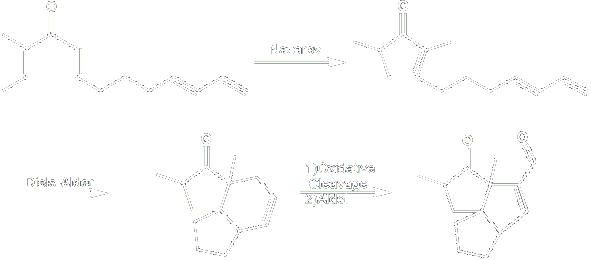
In this tandem process, the Nazarov cyclization followed by an intramolecular Diels-Alder reaction will give a fused 5-6-5 tricyclic compound. Upon further manipulations the desired 5-5-5 tricyclic compound will be obtained.
|