Tiff Ulmer

What you should know
Name:
Tiffany J. Ulmer
e–mail:
Goes by:
Hillary
Status:
PhD Candidate
Bachelor’s Degree:
BSc Honours Chemistry
University of Regina
Hails from:
Regina, Saskatchewan
Often heard saying:
“”
Lab SounDtrack Tune O’ Choice:
“” –
Cheers for:
Edmonton Oilers
Recent Publication:
THe Rest of the Group
All About Me
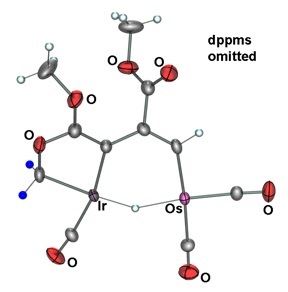
Tiffany’s new research focus is on heterobinuclear complexes and how metal-metal cooperativity invokes distinct reactivity patterns in these complexes, such as using these heterobinuclear complexes as models for mixed-metal Fischer-Tropsch (FT) catalysts. In FT chemistry, methylene groups on the catalyst surface are pivotal intermediates in the conversion of synthesis gas (CO + H2), obtained from coal or natural gas, into hydrocarbons such as “clean” fuels and petrochemical feedstocks. Using Ir/Os diphosphine-bridged complexes, transformations occurring at these complexes can be used as models for the individual steps occurring on the catalyst surface. The Ir/Os system provides strong metal-carbon bonds and, in their low oxidation states, are prone to carbon-hydrogen bond activation. The key methylene-bridged precursor to this chemistry (2) will be generated as shown in Scheme 1 by reaction of the species 1 with diazomethane. Reaction of 2 with C2 fragments such as olefins, allenes, and alkynes should favour an insertion reaction into the methylene-bridge to generate Cn-bridged species. By retaining the growing hydrocarbyl fragment (due to strong metal-carbon bonds), the Ir/Os system could provide a very conducive system in the mechanistic details of FT chemistry.
Ir/M (M = Ru. Os) bimetallic systems are of interest due to their reactivity with strong metal-carbon and metal-hydrogen bonds. These metals, being prone to C–H activation, provide good mechanistic insight into important industrial processes that are often heterogeneous. Motivation for studying these systems also includes the potential to turn inexpensive chemical feedstocks into value-added products.


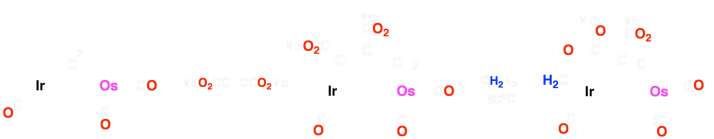
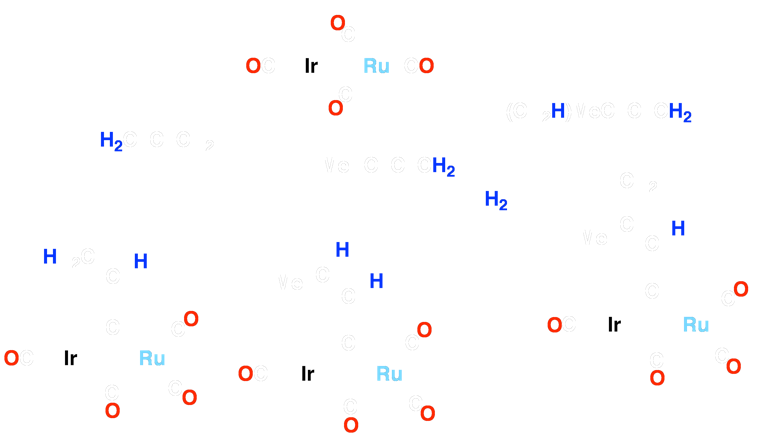
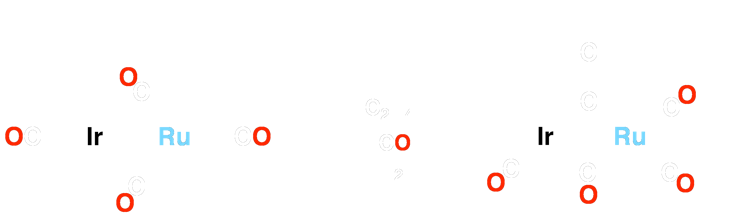
The Ir/Os (1) system can form carbon-carbon bonds via alkyne insertion and carbon-oxygen bonds, generating hydrocarbyl fragments that bridge both metal centers (2 and 3). The Ir/Ru system (4) has been shown to achieve up to three carbon-hydrogen bond activations (5-7) when reacted with cumulenes! Based on preliminary results, complex 8 demonstrates the ability to activate two geminal C–H bonds of ethylene!



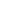
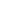

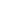
Scheme 1 – Insertion Into Ir/Os System
Scheme 1 – Insertion Into Ir/Ru System
Scheme 1 – Geminal Activation