Matt Zamora

What you should know
Name:
Matthew Thomas Zamora
e–mail:
Goes by:
Zeebot
Tech Support
Status:
PhD Candidate
Bachelor’s Degree:
BSc Honours Chemistry
Mount Allison University
Hails from:
Moncton, New Brunswick
Often heard saying:
“Done and Done...and I mean DONE!”
Lab SounDtrack Tune O’ Choice:
“Then Came the Last Days of May” – Blue Öyster Cult
Cheers for:
Calgary Flames
Recent Publication:
–Zamora, M.T.; Ferguson, M.J.; McDonald, R.; Cowie, M. Dalton Trans., 2009, 7269–7287.
THe Rest of the Group
All About Me
a)
b)
Figure 1 – Phosphines vs NHCs
Scheme 1 – Carbene–Anchored/Pendent–Imidazolium



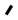
c)
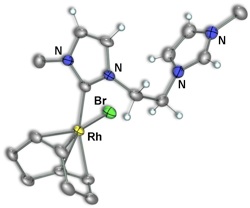
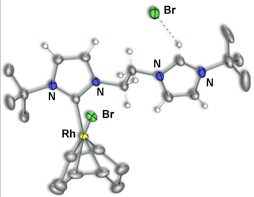
We therefore set out to generate a more extensive series of such pendent species and to use them as synthons for a series of dicarbene–bridged mixed–metal complexes. Initial work focussed on complexes of Rh by deprotonating only one end of the diimidazolium salt precursors (1) to generate species 2 involving the carbene–anchored/pendent–imidazolium moiety (Scheme 2). Subsequent reaction with either carbon monoxide gas or diphosphines can generate the CO (3) or P–P chelated (4–6) analogues, respectively (Scheme 3).
Stepwise
Strategy
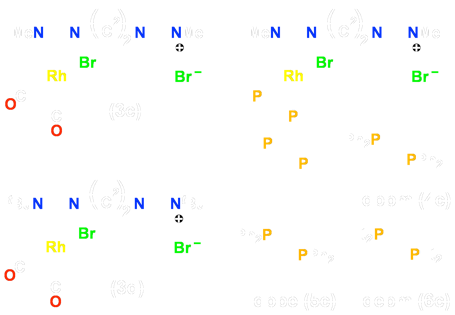
Scheme 4 – Pd Pendent Precursors
Scheme 3 – Modified Rh Pendent Species
In our initial study, we were able to prepare a binuclear dicarbene–bridged complex from a mononuclear, carbene–anchored/pendent–imidazolium precursor by deprotonating the pendent acidic imidazolium hydrogen of 2b using one–half–equivalent of
[Rh(μ–OAc)(COD)]2 yielding the dirhodium target. Attempts to extend this strategy to generate mixed–metal complexes of rhodium using the pendent species above as precursors and using the complexes [Ir(μ–OAc)(COD)]2, or [Pd(OAc)2], containing the basic acetate ligands, all failed. As a result, an analogous series of pendent complexes of palladium were generated as outlined in Scheme 4.
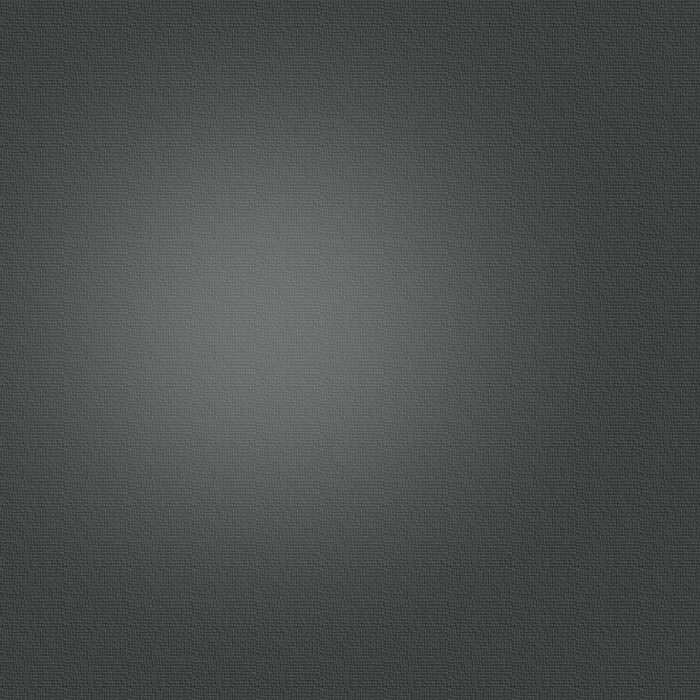
Using this reverse strategy, starting with carbene–anchored/pendent–imidazolium complexes of other metals and using [Rh(μ–OAc)(COD)]2 to perform the deprotonation has allowed us to successfully prepare dicarbene–bridged products of both Pd/Rh (Scheme 5) and Ir/Rh (Scheme 6). Additionally, a number of related Pd/Ir systems (Scheme 7) can be generated by deprotonation of the pendent–imidazolium group by an external base in the presence of [Ir(μ–Cl)(COD)]2. Work is currently underway to examine the reactivity of these bimetallic systems and compare the results to the analogous monometallic systems.
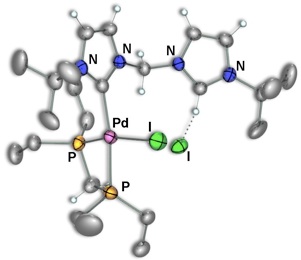
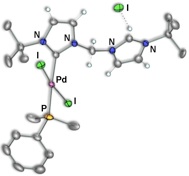
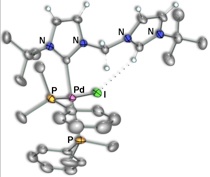
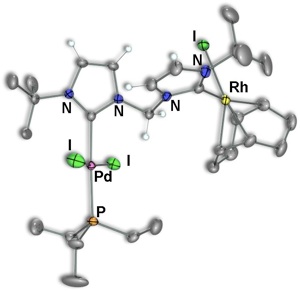
Scheme 5 – Pd/Rh Complexes
Scheme 6 – Ir/Rh Complexes
Scheme 7 – Pd/Ir Complexes
18b
16b
11b
13b
2c
2d
Most reports on NHC complexes involve monocarbenes, however there are a number of reports involving di–N–heterocyclic carbenes (di–NHCs), in which pairs of NHC groups are linked in a number of ways, as replacements for chelating or bridging diphosphines. Our initial study on binuclear di–NHC–bridged complexes concentrated on homobinuclear complexes of rhodium (See Kyle’s section). However we wanted to extend our investigation to complexes in which di–NHC ligands could be used as bridging groups connecting different pairs of metals. For the rational generation of mixed–metal species it appeared that stepwise incorporation of the different metals was the most straightforward strategy. A series of carbene–anchored/pendent–imidazolium salts of the type diagrammed in Scheme 1, appeared ideal for this purpose.
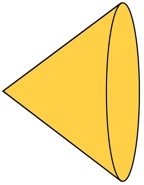
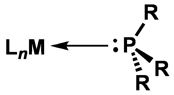
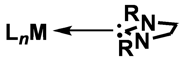
N–carbenes (NHCs) have emerged as versatile ligands in organometallic chemistry, and offer a useful alternative to the ubiquitous phosphine ligands. Although these carbene ligands are considered to have bonding properties similar to those of trialkyl–phosphines their steric properties differ significantly; whereas phosphines are often described as conical, NHC ligands having an unsaturated backbone are more planar, having a slimmer, less sterically hindered axis perpendicular to the carbene ring plane (Figure 1a). This quasi two–dimensional shape is evident in square–planar complexes of NHCs in which the NHC plane is usually perpendicular to the metal coordination plane. Considering this comparison, we wanted to investigate the reactivity of complexes which involve the replacement of the phosphine moieties in our commonly–used bisdiphenylphosphinomethane (dppm) ligands (FIgure 1b) with NHC groups purpose (Figure 1c).
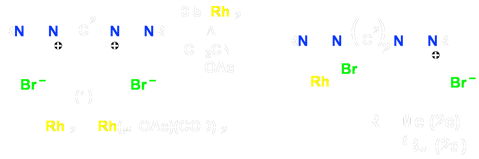
Scheme 2 – Initial Rh Pendent Analogues







click HERE and type the Konami Code if you want any sort of chance in beating Contra.









