Mike Slaney

What you should know
Name:
Michael Slaney
e–mail:
Goes by:
Slaney
Slanedog Millionaire
Status:
PhD Candidate
Bachelor’s Degree:
BSc Honours Chemistry
University of New Brunswick
Hails from:
Oromocto, New Brunswick
Often heard saying:
“”
Lab SounDtrack Tune O’ Choice:
“Renegade” – Styx
Cheers for:
Edmonton Oilers
Recent Publication:
THe Rest of the Group
All About Me

In the fall of 2006, Mike returned to the University of Alberta to continue his education under the supervision of Dr. Cowie. His current research topics include:
(1) Investigating fluoroolefin activation by exchanging the methyl ligand of [Ir2(CH3)(CO)2(dppm)2] (1) by hydride and silyl groups, which can function as “internal fluoride abstractors”.
(2) Using the previously synthesized depm precursors to investigate their reactivity with fluoroolefins.
(3) Attempting to develop catalytic process for the conversion of tetrafluoroethylene into trifluoroethylene, trifluoroethylene into either cis-difluoroethylene or two isomers of difluoropropene, and
cis-difluoroethylene into 2-fluoropropene.
The activation of strong carbon-fluorine bonds, in order to bring about the selective functionalization of fluoro-carbons, yielding products having applications as pharmaceuticals, pesticides, polymers, and refrigerants, and also for the conversion of environmentally harmful chlorofluorocarbons, represents an important, yet formidable challenge. We have established a new strategy for the selective activation of C-F bonds in bridging fluoroolefin ligands, in which a pair of metals act in a cooperative manner to activate the fluoroolefin substrates. The fluoroolefin-bridged
complex 1 product, readily yields the corresponding fluorovinyl (2 and 3) complexes upon the addition of Me3SiOTf, HOTf, or even water!


Scheme 1 – Reactivity of Fluoroolefin–Bridged Complexes
Complex 2 can in turn convert the fluoroolefin moieties into to the corresponding cis-difluoroethylene (4), or a mixture of 1,2-difluoropropene (5) and 2,3-difluoropropene (6) by reactions with H2 or CO, respectively. Using this strategy, we have also succeeded in transforming tetrafluoroethylene into trifluoroethylene and 1,1-difluoroethylene into 2-fluoropropene.
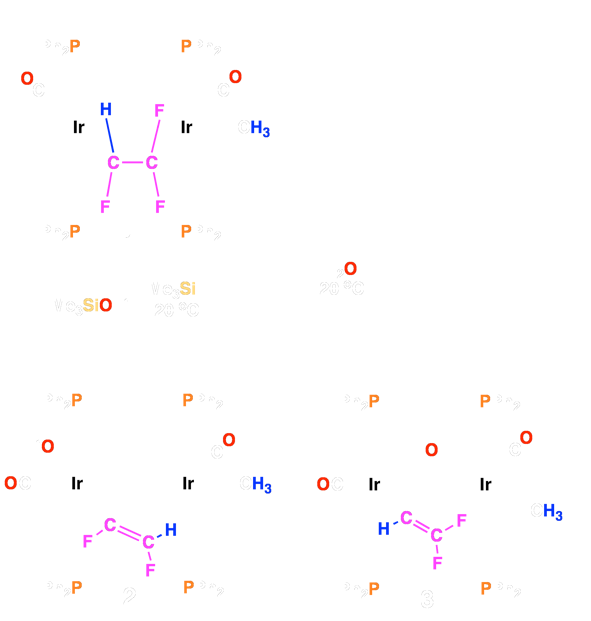






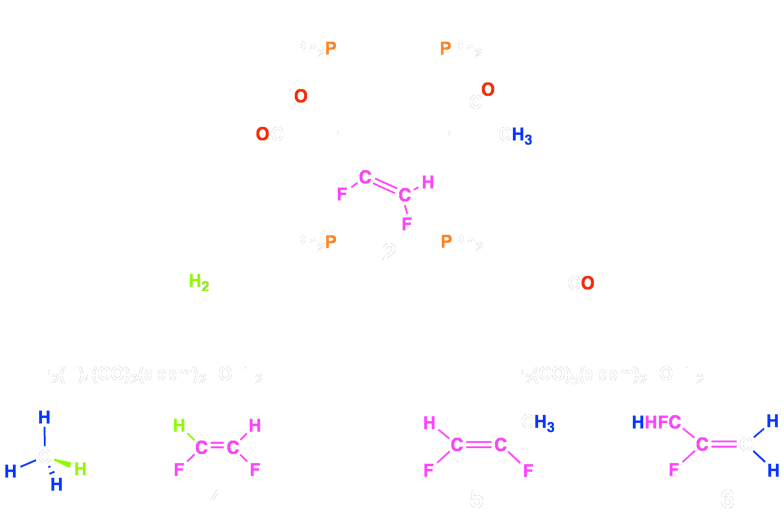


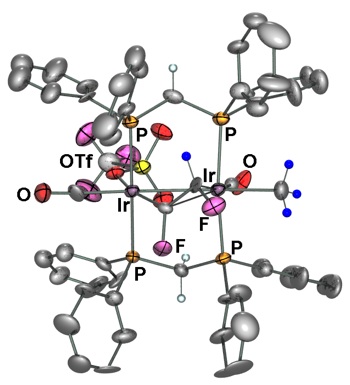
Figure 1 –Fluoolefin–Bridged Di–Ir System
Scheme 2 – Generated Products








