- Home |
- Research: Asymmetric Catalysis - Asymmetric Hydrogenation - Immobile Catalysts - Fuel Cells
Immobilization of Enantioselective Catalysts
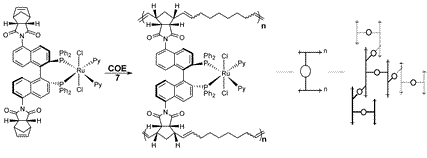
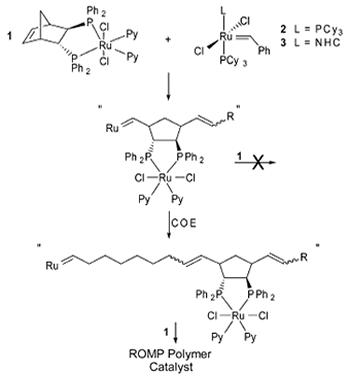 The driving forces for heterogeneous enantioselective catalysis are both economic and environmental in nature. Ideal catalyst systems are highly selective and active, reusable, and do not leach toxic metal into the product. We have previously reported a polymerized hydrogenation catalyst prepared by an alternating ring opening olefin metathesis polymerization (ROMP) between the Norphos compound (1) and cyclooctene (COE) catalyzed by the Grubbs olefin metathesis catalysts 2 or 3. For example, the strained norbornyl group in 1 reacts first with 2 to generate a new Ru-alkylidene that is too crowded to react with another molecule of 1. Instead, the crowded alkylidene reacts with the uncrowded monomer COE to generate yet another alkylidene that is on the end of a C8 spacer. This new, uncrowded alkylidene now reacts with 1, then COE, and so on to produce an alternating polymerized catalyst. After further modifications, this catalyst was effective for the hydrogenation of the test ketone, 1'-acetonaphthone (TON = 500 per run, 83% ee) with 9 reuses without loss of yield or enantioselectivity.
The driving forces for heterogeneous enantioselective catalysis are both economic and environmental in nature. Ideal catalyst systems are highly selective and active, reusable, and do not leach toxic metal into the product. We have previously reported a polymerized hydrogenation catalyst prepared by an alternating ring opening olefin metathesis polymerization (ROMP) between the Norphos compound (1) and cyclooctene (COE) catalyzed by the Grubbs olefin metathesis catalysts 2 or 3. For example, the strained norbornyl group in 1 reacts first with 2 to generate a new Ru-alkylidene that is too crowded to react with another molecule of 1. Instead, the crowded alkylidene reacts with the uncrowded monomer COE to generate yet another alkylidene that is on the end of a C8 spacer. This new, uncrowded alkylidene now reacts with 1, then COE, and so on to produce an alternating polymerized catalyst. After further modifications, this catalyst was effective for the hydrogenation of the test ketone, 1'-acetonaphthone (TON = 500 per run, 83% ee) with 9 reuses without loss of yield or enantioselectivity.
Current research has extended this methodology to develop a new process, the alternating ROMP-assembly of three-dimensional catalyst-organic frameworks. One such three-dimensional catalyst organic framework was reused 25 times for the hydrogenation of 1’-acetonaphthone (TON = 1000 per run, 95% ee) without loss in activity or selectivity, and without detectable leaching of the catalyst metal centre into the product. This is the most reuses of any heterogenized chiral hydrogenation catalyst to date. Other group research in this area includes extending the current systems to enantioselective carbon-carbon bond forming reactions, as well as development of new frameworks using other catalysts and monomers.