- Home |
- Research: Asymmetric Catalysis - Asymmetric Hydrogenation - Immobile Catalysts - Fuel Cells
Asymmetric Hydrogenation
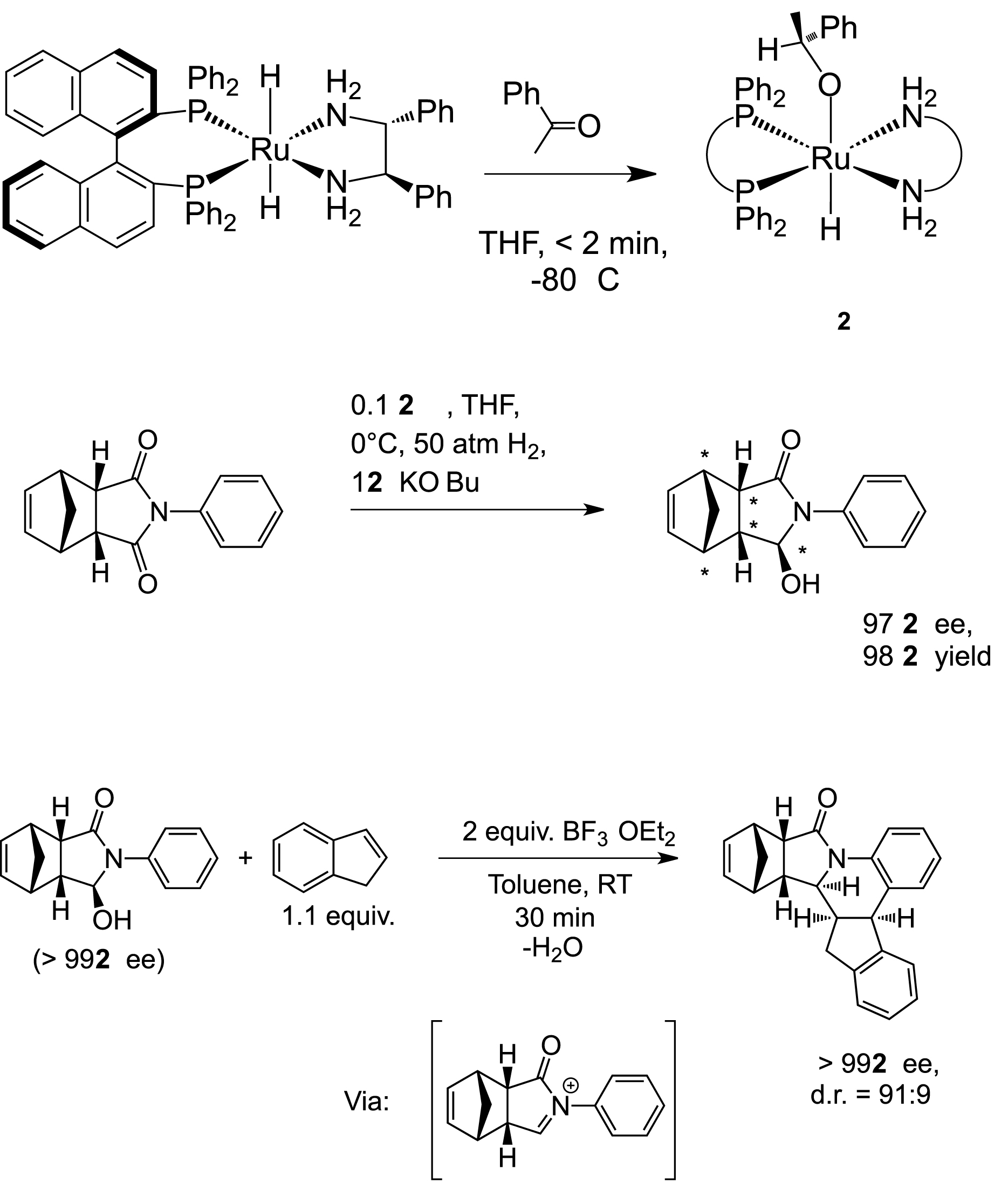 We have carried out a mechanistic study upon the Noyori carbonyl hydrogenation reactions that utilize trans-Ru(diphosphine)(H)2(diamine) and related systems as catalysts. Our research in this area develops organometallic synthesis to prepare, often for the first time, air-, moisture-, and thermally-sensitive catalytic intermediates in the cycle and to study their properties and reactivity. For example, we prepared the cationic η2-H2 species trans-[Ru((R)-BINAP)(H)(η2-H2)((R,R)-dpen)]+ (BINAP = 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, dpen = 1,2-diphenylethylenediamine). The η2-H2 ligand in this species is extremely labile, and to our knowledge, has the shortest H-H bond distance (~0.802 Å based on 1JH-D = 37 Hz) of any dihydrogen compound reported to date. We also prepared and fully characterized the active dihydride catalyst trans-[Ru((R)-BINAP)(H)2((R,R)-dpen)] (1), and found it is a remarkably powerful carbonyl reducing agent. For example, we observed that the bifunctional addition of acetophenone to 1 in THF is complete on mixing at -80°C, and that the net product is the corresponding Ru-alkoxide intermediate 2 (Scheme 1). This unexpectedly high reducing power led us to develop a remarkably low T and P hydrogenation of simple esters. And we developed a new reaction, the enantioselective desymmetrization of meso cyclic imides via mono hydrogenation (Scheme 1). One example of this desymmetrization produced 5 chiral centres (97% ee) in one step. This number was increased to 7 by an electrophilic addition of the corresponding N-acyl iminium ion to indene, thereby creating substantive molecular complexity in only 2 steps. Current research on hydrogenation involves preparing new catalysts via well-defined stoichiometric and catalytic trials, and quite recently, by rapid screening. Objectives include higher turnover numbers, yields, the development of base-free systems, and hydrogenations of amides and related challenging substrates. Efforts are also underway to determine the intimate details of the bifunctional addition.
We have carried out a mechanistic study upon the Noyori carbonyl hydrogenation reactions that utilize trans-Ru(diphosphine)(H)2(diamine) and related systems as catalysts. Our research in this area develops organometallic synthesis to prepare, often for the first time, air-, moisture-, and thermally-sensitive catalytic intermediates in the cycle and to study their properties and reactivity. For example, we prepared the cationic η2-H2 species trans-[Ru((R)-BINAP)(H)(η2-H2)((R,R)-dpen)]+ (BINAP = 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, dpen = 1,2-diphenylethylenediamine). The η2-H2 ligand in this species is extremely labile, and to our knowledge, has the shortest H-H bond distance (~0.802 Å based on 1JH-D = 37 Hz) of any dihydrogen compound reported to date. We also prepared and fully characterized the active dihydride catalyst trans-[Ru((R)-BINAP)(H)2((R,R)-dpen)] (1), and found it is a remarkably powerful carbonyl reducing agent. For example, we observed that the bifunctional addition of acetophenone to 1 in THF is complete on mixing at -80°C, and that the net product is the corresponding Ru-alkoxide intermediate 2 (Scheme 1). This unexpectedly high reducing power led us to develop a remarkably low T and P hydrogenation of simple esters. And we developed a new reaction, the enantioselective desymmetrization of meso cyclic imides via mono hydrogenation (Scheme 1). One example of this desymmetrization produced 5 chiral centres (97% ee) in one step. This number was increased to 7 by an electrophilic addition of the corresponding N-acyl iminium ion to indene, thereby creating substantive molecular complexity in only 2 steps. Current research on hydrogenation involves preparing new catalysts via well-defined stoichiometric and catalytic trials, and quite recently, by rapid screening. Objectives include higher turnover numbers, yields, the development of base-free systems, and hydrogenations of amides and related challenging substrates. Efforts are also underway to determine the intimate details of the bifunctional addition.