- Home |
- Research: Asymmetric Catalysis - Asymmetric Hydrogenation - Immobile Catalysts - Fuel Cells
Fuel Cell Development
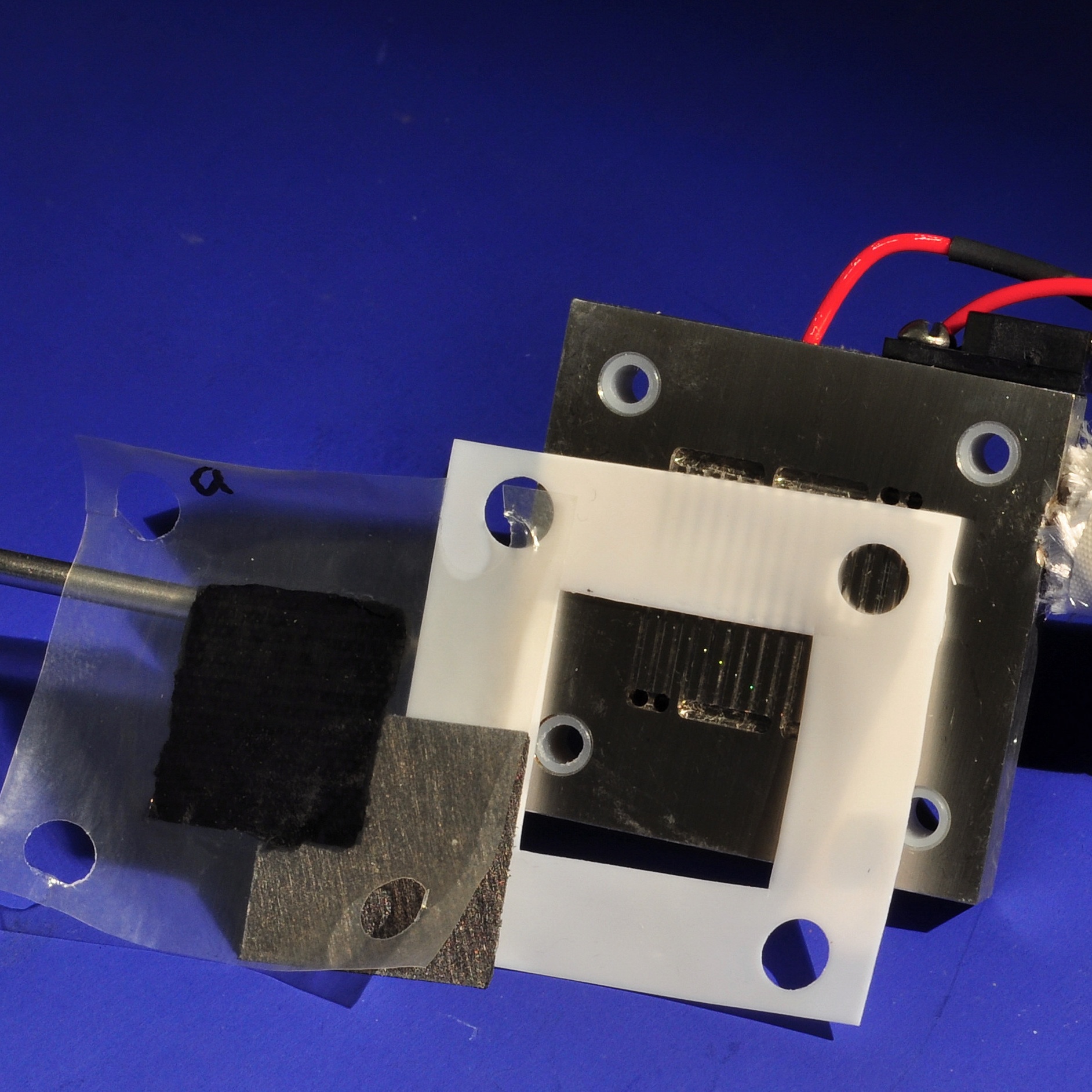
Polymer electrolyte fuel cells are a promising power source for portable electronic devices such as cell phones and laptop computers. The best cell performances are achieved by the hydrogen/oxygen fuel cell. The fuel, hydrogen, reacts at the surface of the anode to produce two protons and two electrons. The electrons flow through an external circuit to the cathode producing electrical work, while the protons travel through a polymer electrolyte membrane that has been chemically functionalized to promote proton transfer. At the cathode, the protons, electrons, and oxygen from air combine to form water as the only byproduct; the net result is a clean, efficient power source that can operate as long as fresh fuel is supplied at the anode and air is supplied to the cathode.
The problems associated with the storage of hydrogen fuel pose a considerable hindrance towards the commercialization of hydrogen fuel cells. Upon mixing with oxygen, hydrogen gas is extremely explosive; providing a considerable safety concern in the event of a fuel tank leak. Additionally, gaseous hydrogen, unless under high pressure, produces too few electrons per unit volume of fuel to be reasonable as a power source. Use of liquid hydrogen requires cryogenic equipment and high thermodynamic demands associated with its formation.
Alternative storage strategies ranging from the adsorption of hydrogen onto high surface area materials, and the decomposition of hydrated solids, or using an alternative reducing agent as a fuel source are under investigation. The characteristics of current hydrogen-containing solids are improving, but they have yet to reach the desired targets to be utilized as fuel reservoirs. Considerable effort has also been put into the development and understanding of fuel cells that run on alcohols; as they are liquids under ambient conditions making them easier to store and transport, and they contain more electrons then gaseous hydrogen per unit volume of fuel.
Our research is focused on the heterogeneous electro-catalytic oxidations of alcohols over supported and unsupported noble metal catalysts such as platinum, ruthenium, and their composites. Central to this project is the use of methanol, ethanol, and secondary alcohols such as 2-propanol as fuels. Methanol is readily available and has promising electrochemical activity. Ethanol from biostock is a renewable fuel. The electro oxidation of 2-propanol over platinum in alkaline electrolytes produces high current at potentials considerably lower then those required to oxidize methanol, currently the benchmark in alcohol fueled fuel cells. It is generally believed that the oxidation of 2-propanol over platinum at low potentials produces acetone, protons, and electrons, which proceeds by a mechanism that does not produce strongly adsorbed species that poison the catalyst. In contrast, the electro-oxidation of methanol produces carbon monoxide, a platinum poisoning species that results in performance losses due to anode polarization. The produced acetone from 2-propanol oxidation can be catalytically hydrogenated by catalysts developed in this lab, effectively constructing a rechargeable indirect hydrogen fuel cell that uses a secondary alcohol as the hydrogen transport medium.
Another goal within this project involves the reduction of platinum content by using a catalyst support. The use of platinum as an electro-catalyst is commercially prohibitive due to its high cost, thus the reduction of platinum content is a key concern for cost effective commercialization of fuel cells. The preparation of a nickel supported platinum electro-catalysts, where the catalyst consist of an atomic layer of platinum supported on a nickel core would considerably decrease the material cost of fuel cell electrodes, while keeping the activity of a platinum electro-catalyst. Recent results have shown that appreciable currents can be obtained from low surface loadings of platinum on nickel wire gauzes, and further research is being conducted to optimize the synthesis of this material for use as a fuel cell electro-catalyst.
Researchers in this project become proficient in qualitative electrochemistry, utilizing techniques to studying half-cell reactions, such as cyclic voltammetry and static potential oxidations. Surface modification techniques are implemented to synthesize electro-catalysts with high surface areas; ranging from pure noble metals, to surface and bulk modified composites by electrochemical and chemical depositions. The fabrication of polymer bound nanoparticle catalyst layers, construction of prototype fuel cells with liquid or solid polymer electrolytes, and techniques used in evaluating cell performance characteristics are learnt.