Research Interests
Research in our group lies within the general realm of synthetic inorganic and polymer chemistry, with particular focus on developing new routes to industrially relevant semiconducting and electronically insulating materials. Along the way, we aim to gain fundamental insight into the nature of bonding and reactivity across the Periodic Table, with an emphasis on p-block element chemistry. Consequently, researchers in the Rivard group utilize a variety of advanced inorganic/polymer synthesis and characterization techniques, including quantum mechanical (DFT) methods.
Donor-acceptor stabilization as a general route to novel bonding environments and for the preparation of next generation advanced materials under mild conditions
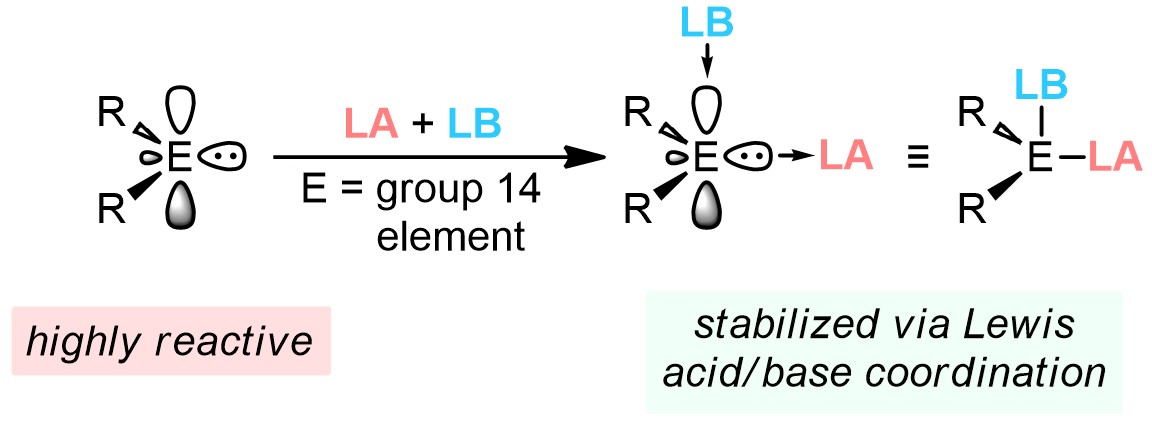
We use the cooperative abilities of Lewis acids (LAs) and Lewis bases (LBs) to trap/stabilize various reactive main group species with unusual bonding arrangements, often involving low-oxidation state p-block elements. A highlight in this area is our preparation of donor-acceptor complexes of "inorganic methylenes", EH2 (E = silicon, germanium, and tin). Rather than being simple laboratory curiosities, these isolable/bottleable hydride complexes can act as useful molecular precursors for the thermal deposition of industrially relevant semiconductors at record-low temperatures (often below 100 °C); typically, bulk Si and Ge are prepared at temperatures > 550 °C.
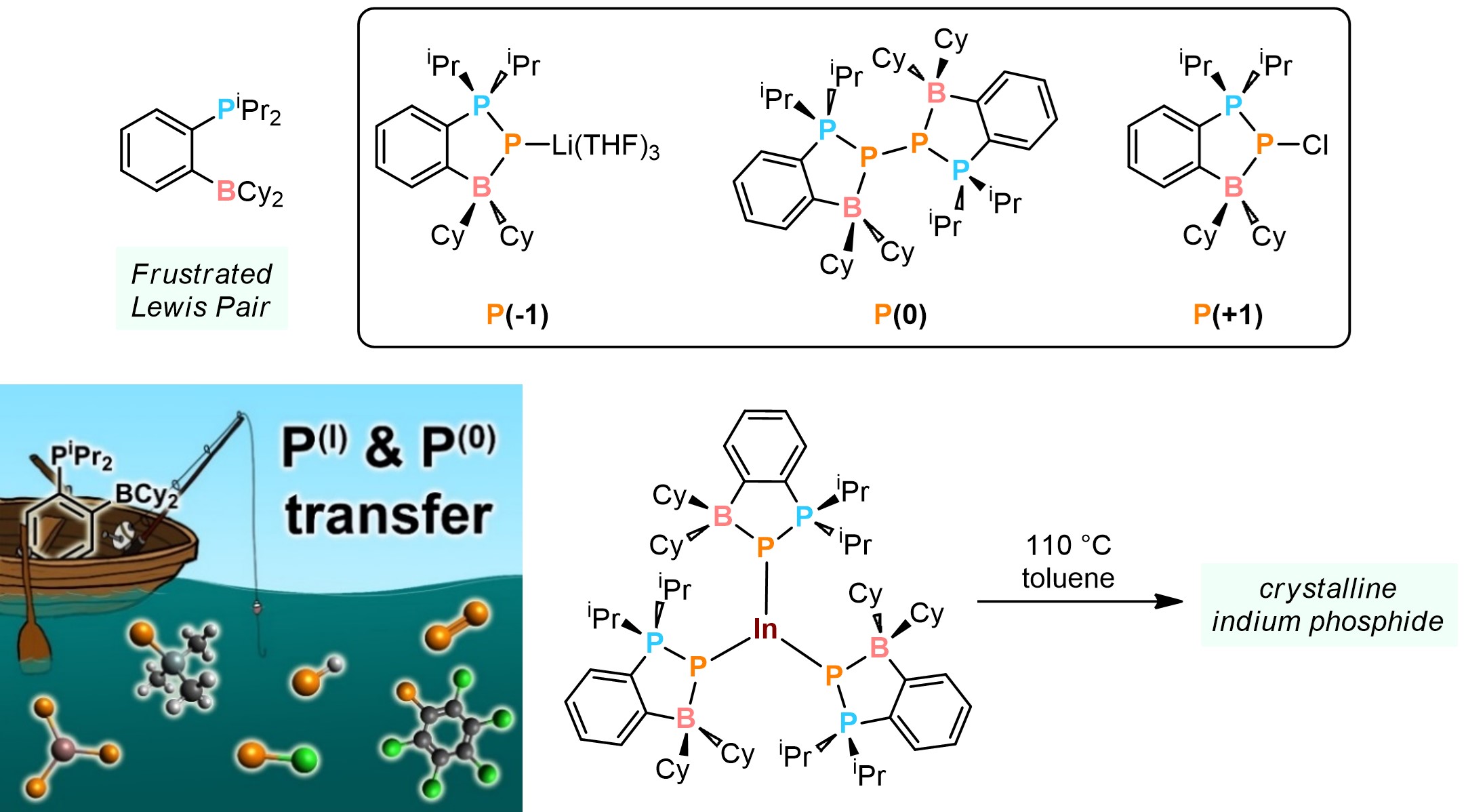
We explore chelating frustrated Lewis pairs (FLPs) for the stabilization of low-valent group 15 element fragments. Recently, we showed that FLPs can stabilize phosphorus in its exotic 1 (PLi), 0 (P2), and +1 (PCl) oxidation states. These species can be used to deliver phosphorus-containing fragments to organic substrates, making them useful tools for the synthesis of novel organophosphorus ligands. Moreover, these FLP-adducts can be used as P-atom sources for the synthesis of inorganic semiconductors like indium phosphide, avoiding the need for harsh P-sources like the highly toxic PH3 gas.
More information:
Inorg. Chem. 2020, 59, 1099611008.
Angew. Chem. Int. Ed. 2021, 60, 228231.
Dalton Trans. 2023, 52, 16021607.
Angew. Chem. Int. Ed. 2023, 62, e202218587.
Design of novel N-heterocyclic carbene (NHC) and N-heterocyclic olefin (NHOs) ligands
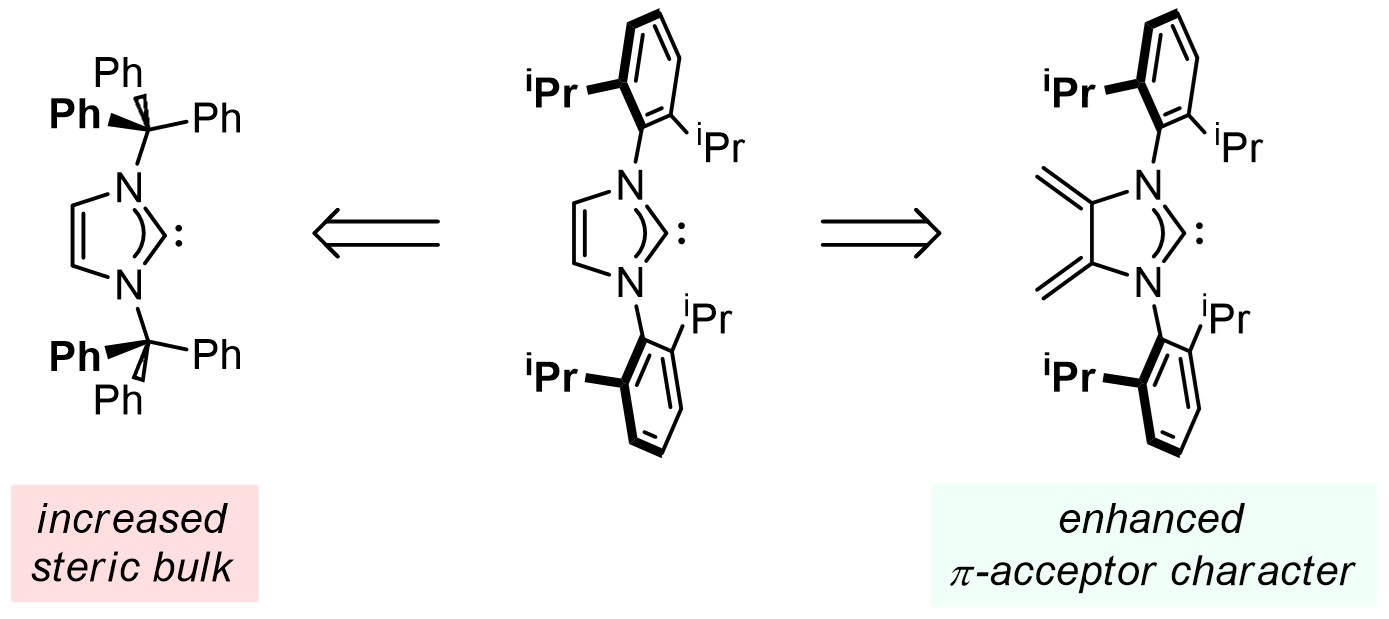
N-Heterocyclic carbenes (NHCs) are widely used ligands in catalysis and for the stabilization of fundamentally interesting low-coordinate species. We are developing novel NHC ligands with unique steric and electronic properties: such as extreme steric bulk for the isolation of reactive one-coordinate metals and the synthesis of new "diene-functionalized" NHCs for the incorporation of this ligand into polymers via its reactive diene unit, both towards the goal of obtaining better catalysts.
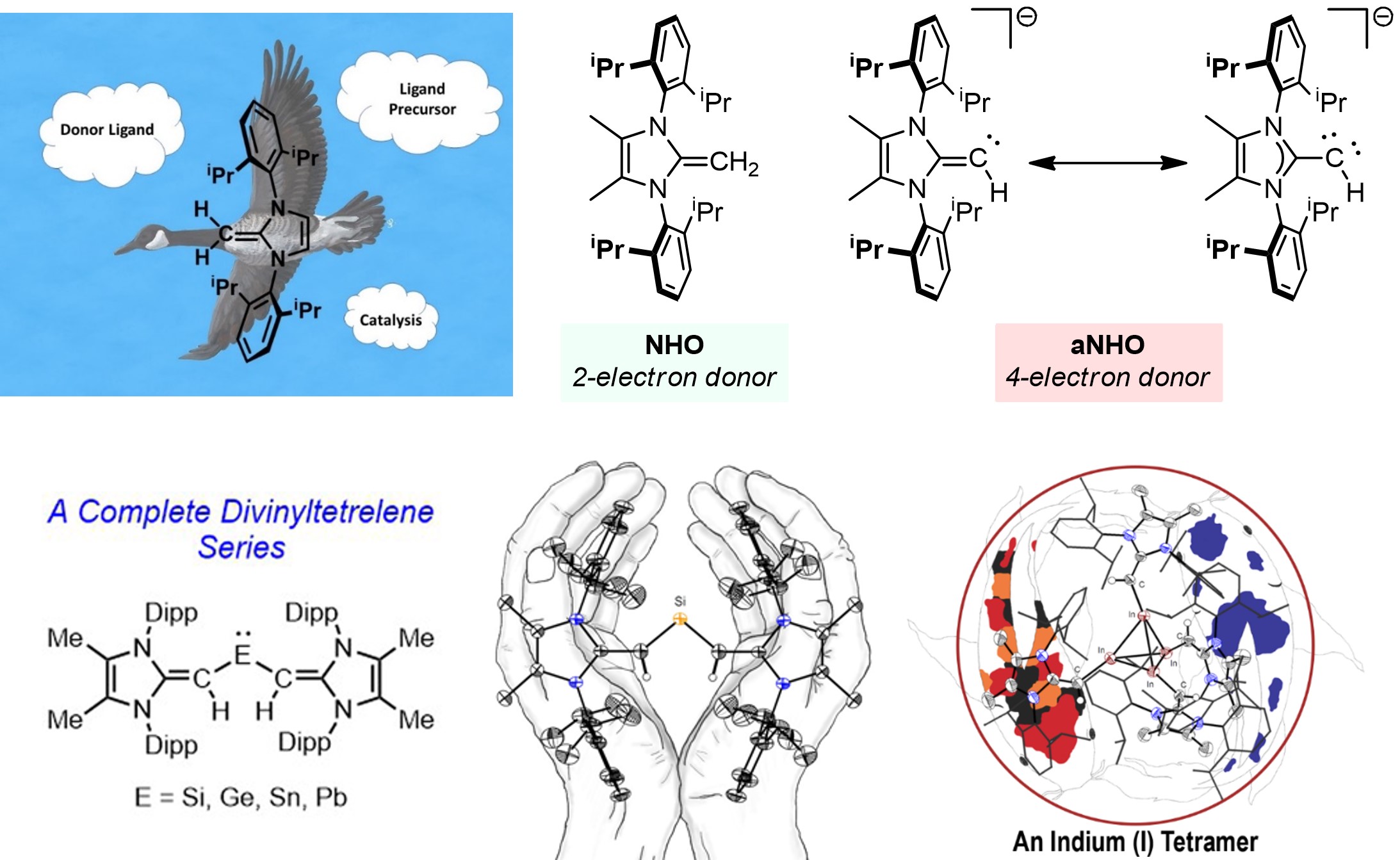
N-Heterocyclic olefins (NHOs) are similar to NHCs but tend to be weaker s-donating ligands; in many cases, this makes them superior to NHCs for organocatalysis and \olefin polymerization applications as they do not bind to the final products as strongly. Much of our recent work focuses on the use of anionic NHOs (aNHOs) to stabilize reactive molecules. In their anionic form, NHOs can act as 4-electron donors, while keeping substantial steric bulk about the coordinate site. We have used aNHOs to stabilize the first acyclic tetrelenes (aNHO)2E: (E = Si, Ge, Sn, Pb) and the indium(I) tetramer [(aNHO)In]4; we hope to introduce this ligand to other areas of the Periodic Table as examples of sterically- and electronically-modifiable analogues of imines (R2C=N-), which are used often as ligands in catalysis.
More information:
Acc. Chem. Res. 2017, 50, 20172025.
Chem. Commun. 2018, 54, 483486.
Chem. Sci. 2019, 10, 64766481.
Chem. Eur. J. 2021, 27, 85728579.
Chem. Commun. 2023, 59, 29032906.
Polymers containing p-block elements
Polymers are a ubiquitous and essential part of modern living, and as a result, brand-name polymers such as Nylon, Teflon, and Kevlar are now entrenched in our vocabulary. Compared to these common organic polymers, polymers containing heavier p-block elements have received less attention. Our research aims to create novel polymers incorporating main group elements, both within the polymer backbone and as pendent groups. We have recently prepared polyacetylenes bearing electronically active p-block elements as side groups to access new polymers with very low HOMO-LUMO gaps for solar cell applications (as low as 1.5 eV; silicon metal has an Eg of 1.1 eV), improved air stability, high degrees of electrical conductivity, on-off optical switching in telecommunications region of 1500 nm, and sufficient solubility to allow for the solution-phase processing of polyacetylenes (typically insoluble materials). As shown below, the high electron density along the polymeric olefin backbone leads to interesting surface optical effects, much like what is observed for metals (e.g., Au).
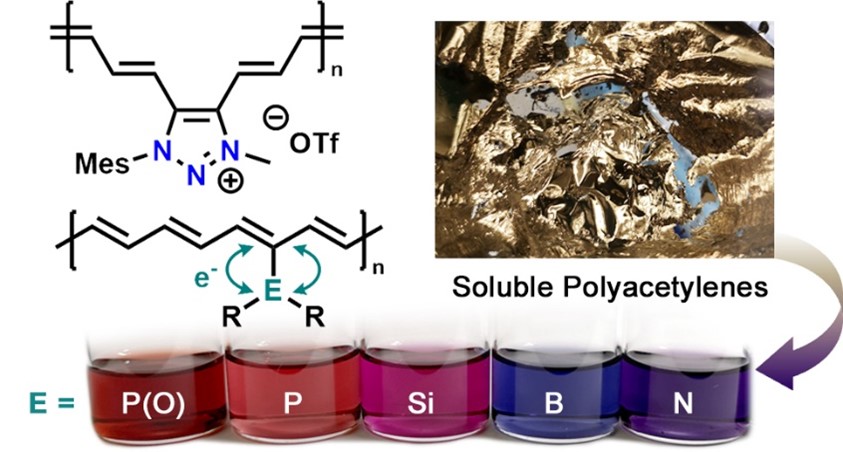
In another recent advance in our laboratory, we have prepared polymers with Ge-Ge bonds along the polymer chain, polygermanes, that are designed to liberate the known semiconductor Ge at room temperature in the presence of UV light. As shown below (right), these polygermanes can be used for the photolithography ("light writing") of crystalline Ge, a process that is of substantial interest to the nanoelectronics industry where photolithography is commonly used to make patterned circuits.
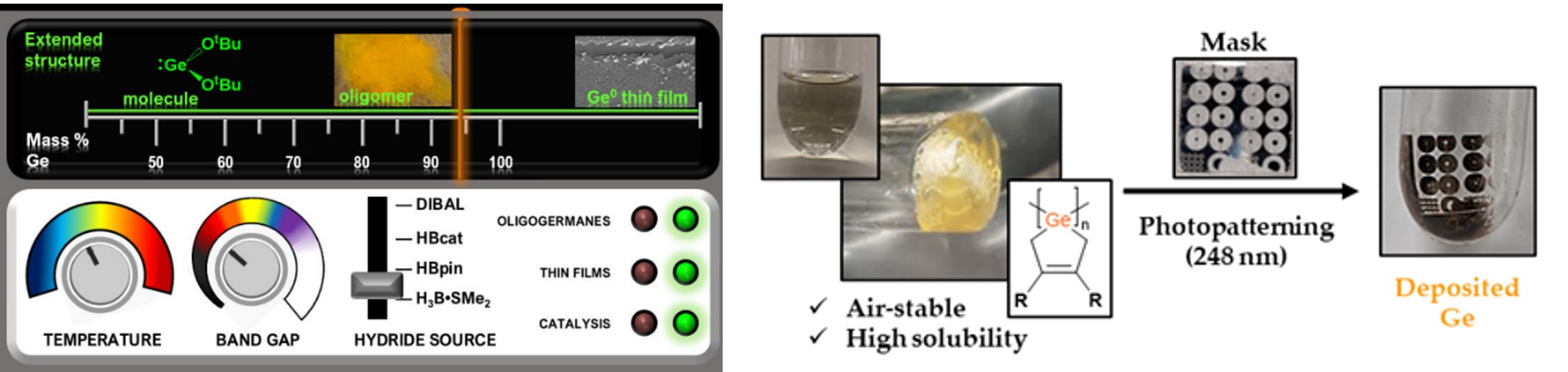
More information:
Angew. Chem. Int. Ed. 2021, 61, e202114586.
Dalton Trans. 2021, 50, 1768817696.
Chem. Commun. 2023, 59, 68496852.