Washing and drying of organic solution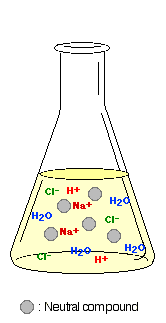
-treatment before
distillation-
-
- After performing several liquid-liquid
extractions using aqueous base or acid, the organic solution
is contaminated with inorganic impurities (such as NaCl,
NaOH or HCl), as well as "wet" with water.*
-
- *Even though you use an organic solvent
which is immiscible with water for extraction, the two phases
dissolve in each other to some extent. (For example, 100 mL of
dichloromethane can dissolve 1.32 g of water at 25oC)
- You need to remove the inorganic
impurities and water from the organic solution before
distillation.
- Washing
is the step of removing inorganic impurities.
-
- Drying
is the step of removing water.
Organic Web Chem University of Alberta