Research –
The activation of very strong carbon–fluorine bonds, in order to bring about the selective functionalization of fluorocarbons, yielding products having applications as pharmaceuticals, pesticides, polymers and refrigerants, and also for the conversion of environmentally harmful chlorofluorocarbons, represents an important, yet formidable challenge.
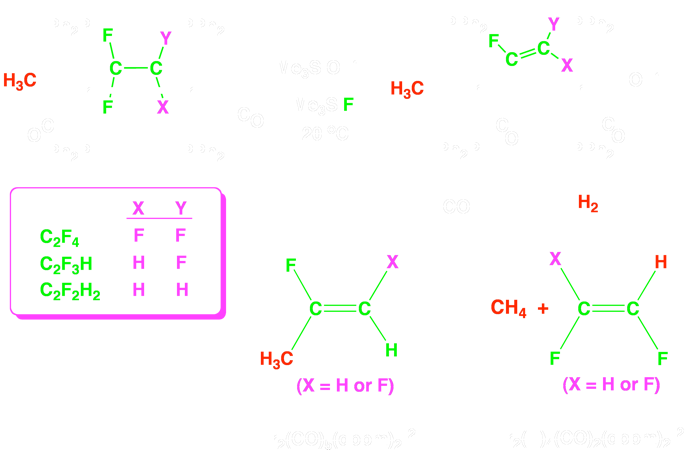
We have established a new strategy for the selective activation of C–F bonds in bridging fluoroolefin ligands, in which a pair of metals act in a cooperative manner to activate the fluoroolefin substrates.
The fluoroolefin–bridged [Ir2(CH3)(CO)2(µ–fluoroolefin)(dppm)2]+ (dppm = µ–Ph2PCH2PPh2) products, shown in the scheme, readily yield the corresponding fluorovinyl complexes, which can in turn be converted to the corresponding fluoroethylene or fluoropropene products by reactions with H2 or CO, respectively.
Using this strategy, we have succeeded in transforming tetrafluoroethylene into trifluoroethylene, trifluoroethylene into either cis–difluoroethylene or two isomers of difluoropropene, and cis–difluoroethylene into 2–fluoropropene.
Currently, we are attempting to optimize the reactivity of this system, by using smaller and more basic alkyl diphosphine ancillary ligands and by exchanging the methyl ligand by hydride and silyl groups, which can function as “internal fluoride abstractors”. We are also attempting to develop catalytic processes from the above stoichiometric reactions.
For more information, see Mike’s work
C–F Activation of Fluoroolefins



C–F Activation of Fluoroolefins



Breaking Down CFCs