|
|
|
|
|
|
|
|
|
|
|
Previous research fell into two fairly broad categories (details of which are provided below):
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Details to be posted shortly.
It is well-known that homonuclear diatomic molecules (e.g. N2 or
O2) do not have permanent dipole moments in the ground electronic
state. Therefore, the gas does not exhibit vibration-rotational absorption in
the infra-red region as hetero-polar diatomics do. However, there are weak
continuum-type transitions due to translational, rotational, and vibration-
rotational transitions caused by transient dipoles that are induced during
binary collisions. The latter is generally called collision-induced absorption
(CIA) or supermolecular absorption. The understanding of CIA is important for
the study of radiative transfer in the Earth's atmosphere.
The CIA has been determined for O2 and N2
for N2-N2, O2-O2,
O2-N2, and N2-O2 pairs where
in each instance the first molecule listed in assumed to make the fundamental
transition. For each of these dimers, the isotropic
interaction (anisotropy is ignored) is modeled by a Lennard-Jones potential.
Using the LJ potential and the multipole-induced dipole functions, the collisioninduced absorption can be determined. Please see the listed references for
a complete discussion of the theory.
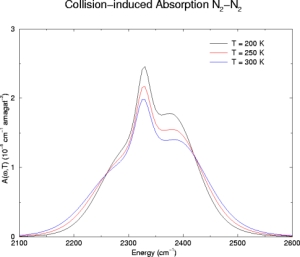
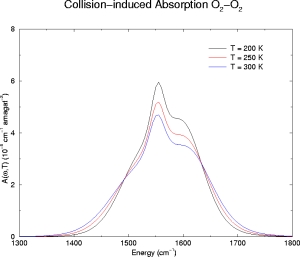
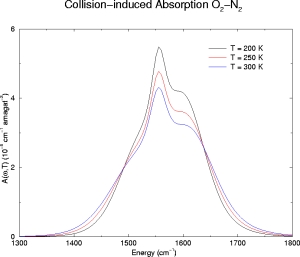
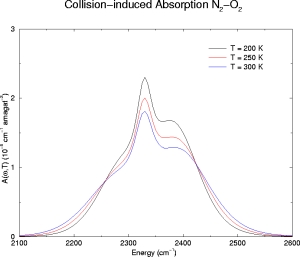
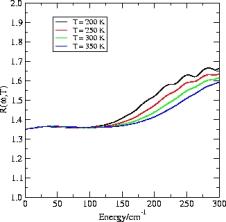
Below we have illustrative plots for T = 200K, 250K and 300K. More importantly, we
make available data for the absorption coefficient (in units of cm-1
amagat-2)
for these various pairs over a wide temperature range. The spectra have been computed
every 10 K from 190 - 310 K.
For N2, the
fundamental band has been computed from 2100-2600 cm-1 and
for O2, the fundamental has been computed from
1300-1800 cm-1. The spectrum has been computed every 5
cm-1 within these energy ranges.
The data is available in two formats:
Currently, these are the only results we have available. If there are any other
species that you are interested in, please feel free to contact us as we
may be able to help.
Write to Professor R.H. Tipping or
Dr. A. Brown and we will try
to get back to you as soon as possible.
N2-N2
O2-O2
NOTE: The O2-O2 calculation includes a short-range contribution for the L = 3 mechanism of the form m 3exp[-(R - s)/(0.11s)] where m3 = 5.0x10-5.
O2-N2
and N2-O2
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Translation-rotation correction factor
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Home | Group | Research | Teaching | Publications | Presentations | News | Chemistry Home | U. Alberta Home |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
This page maintained by
alex.brown@ualberta.ca Last updated August 8, 2003. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||