|
High Resolution Spectroscopic Characterization of Chiral Recognition We combine a molecular beam with rotational and ro-vibrational spectroscopy to investigate chiral molecular complexes generated in a supersonic expansion. The non-invasive, very low temperature environment of a pulsed expansion provides an ideal ‘laboratory’ to prepare chiral molecular complexes. These studies provide detailed information of strucrtures and stability ordering of the complexes and allow us to examine chiral recognition forces at play on the molecular level. |
|
Ref: N.Borho and Y. Xu, Angew. Chem., 2007, 119, 2171, 2326 – 2329; Angew. Chem. Int. Ed., 2007, 46, 2123, 2276-2279. (VIP paper, Published Online: 17 Nov 2006, DOI: 10.1002/anie.200603809).
This article is featured on the COVER of Angew. Chem. Int. Ed., Volume 46, issue 13, March 19, 2007. |
|
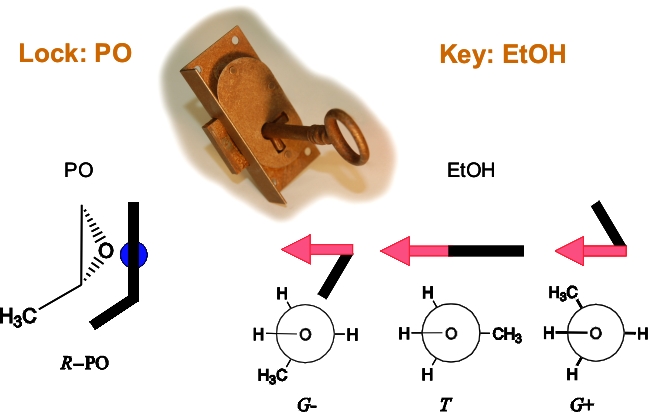
Propylene oxide (PO) is a rigid chiral molecule and can be pictured as the 'solid lock' in our lock-and-key metaphor.
It can only act as a proton acceptor in a classic O-H···O hydrogen-bond. EtOH, on the other hand, can adopt three different conformations:
gauche+ (G+), gauche- (G-) and trans (T) with the torsional angle HOCC at +60, -60 and 180 degrees, respectively. Although it does
not have a permanent chiral centre, the G- and G+ conformers are enantiomer to each other, related via helical chirality.
They can interconvert through a tunnelling process which is expected to be quenched in the PO···EtOH hydrogen-bonded molecular adduct.
Therefore, the G+ and G- configurations are locked in their respective spatial configurations. This phenomenon is termed transient chirality. |
|
Copyright © 2003 Dr. Xu's lab
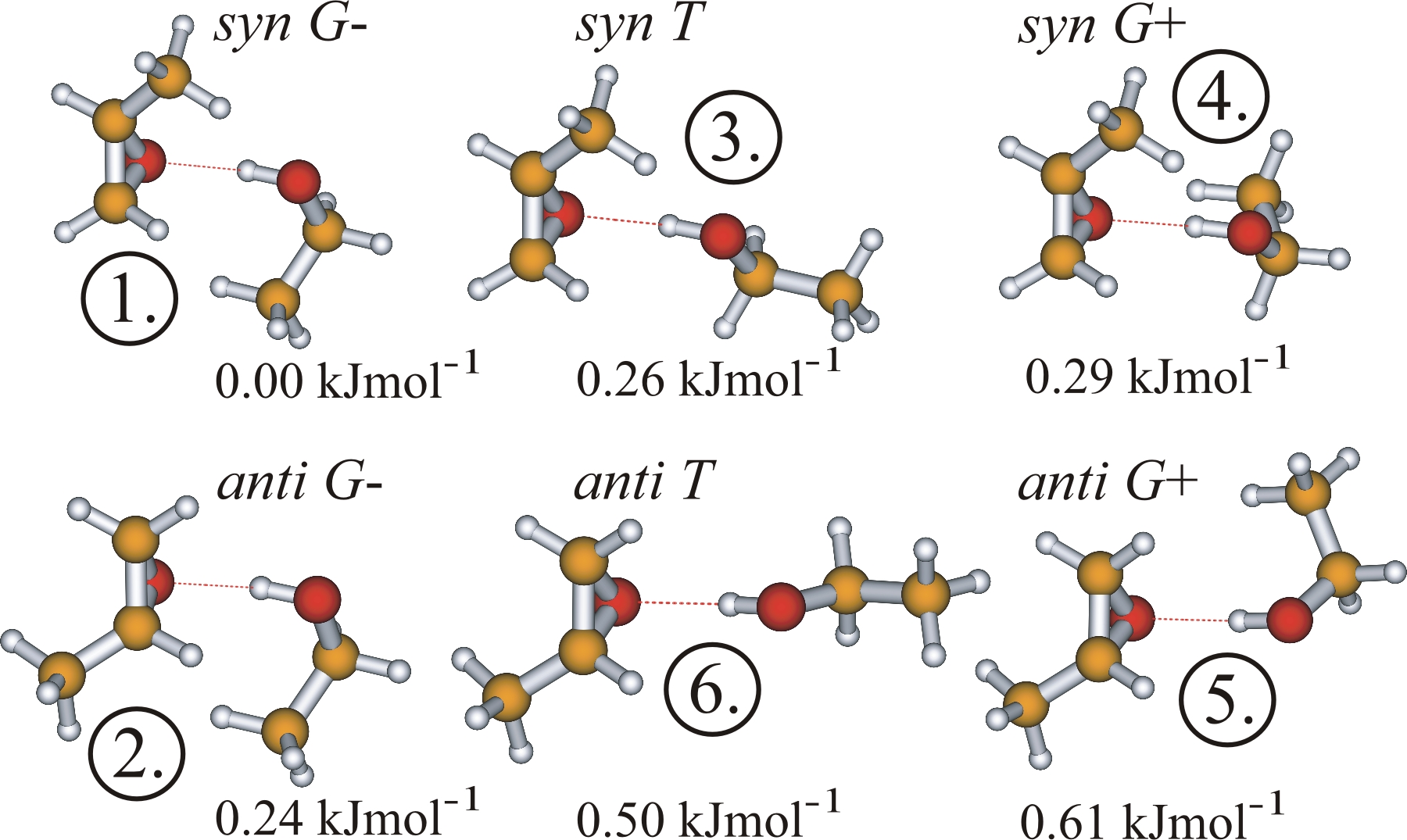 The three EtOH configurations, i.e. G+, G-, T, can bind to the PO oxygen acceptor either on the same side of the
PO methyl group (syn) or on the opposite side (anti). Consideration of both R and S enantiomers of PO leads to 3x2x2 = 12
different structures, forming six pairs of enantiomers. Since each R–PO···EtOH complex has a mirror image, S–PO···EtOH,
and both give rise to the same rotational spectrum, only one enantiomer, namely R–PO, is used.
The three EtOH configurations, i.e. G+, G-, T, can bind to the PO oxygen acceptor either on the same side of the
PO methyl group (syn) or on the opposite side (anti). Consideration of both R and S enantiomers of PO leads to 3x2x2 = 12
different structures, forming six pairs of enantiomers. Since each R–PO···EtOH complex has a mirror image, S–PO···EtOH,
and both give rise to the same rotational spectrum, only one enantiomer, namely R–PO, is used.