Proteins are the workers and the main communicators in a cell. Therefore, understanding which proteins are "talking" to each other (protein-protein interactions) helps us understand what changes are occurring in the cell.
Additionally, just as people give items to each other (food, gifts, work documents), the small molecules that are "given" to a protein (such as phosphorylation, glycosylation, etc) can change what each protein will do.
If we want to investigate interactions and modifications at a single protein level, how can we affect only one protein in the midst of thousands?
Bioorthogonal chemistry allows us to modify a single protein or other biomolecule without affecting any others. This is because we use reactions that are completely foreign to the cell, and we incorporate unnatural groups that direct the reaction to a particular molecule or protein. Yet, the number of bioorthogonal reactions that can be used in living cells are few. So, the Williams lab is interested in identifying new ones.
Most bioorthogonal reactions involve two reaction partners. We are developing new reactions that include a third component - a catalyst. The catalyst allows for the two other partners to be present and at equilibrated concentrations before the reaction is initiated, and it also provides access to a wider variety of reaction types.

Think of proteins in the cell like people in a busy public square. We want to know who talks with who, what they talk about, and how that changes where they go and who they talk to next.
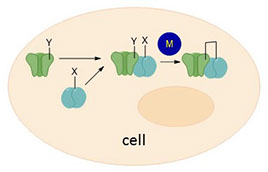
A catalyzed bioorthogonal reaction between two proteins. M = catalyst, X and Y are unnatural groups that direct the catalytic reaction
These new catalyzed bioorthogonal reactions will allow the linking of two species in the cell. These could be two proteins which were already close and "talking" to each other, or it could be a sugar that causes the protein to change its activity (i.e. mimic of glycosylation).
Then, we can study what that change did to the cellular communication and activity network, or we can identify who was close to whom at the time of catalyst addition.
Sometimes you need to know who to speak to in order to get where you want to go. The same is true for drug design.
We want to know which protein(s) are critical gatekeepers to trigger neuroprotective responses without also triggering other unwanted responses. In order to do this, we must understand the full communication network (protein signaling cascade) that is triggered when a neurotrophic natural product interacts with the cell. Many neurotrophic molecules are quite different, so we hypothesize that there will be different protein signaling networks activated by different types of molecules. This should provide us with new players in the neuroprotective response network.
Once we have identified key proteins, we can begin designing drugs that target them, hopefully providing new therapeutic options and strategies.
Please contact Florence Williams by email for further information.
Previous