Glucose Hemi-Acetal and Acetal (Glycoside) Formation:
Some Common Concepts in Carbohydrate ("Sugar") Chemistry
Carbohydrates
Saccharides
Prefixes and Suffixes
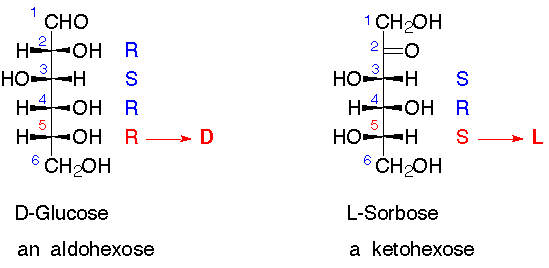
D and L Sugars
Glucose Hemi-acetal Formation
Glucose Acetal (Glycoside) Formation
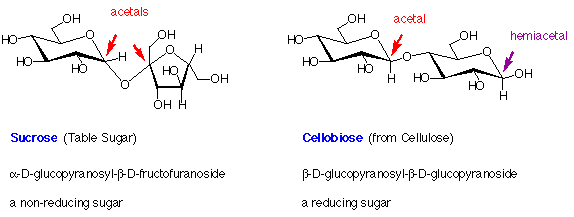
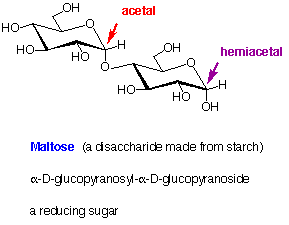
Examples of Disaccharides
Download pdf
1. Carbohydrates:
- (Carbon + Hydrate) are molecules with three or more carbons and a general formula that approximates CnH2nOn
2. Saccharides:
- are carbohydrates or sugars.
- Monosaccharides have one sugar moiety
- Disaccharides have two sugars linked together
- Trisaccharides have three
- Tetrasaccharides have four etc...
- Polysaccharides have an indeterminate number which can be hundreds of thousands or more.
3. Prefixes and Suffixes:
- The suffix (ending) for sugar names is: -ose
- The prefix defines the number of carbons:
- Triose (3 carbons)
- tetrose (4 carbons)
- pentose (5 carbons)
- hexose (6 carbons) etc
- A further prefix defines the types of carbonyl group in the sugar:
- aldo- (aldehyde) or
- keto- (ketone)
- for example glucose (shown below) is an "aldohexose" whereas fructose is a "ketohexose"
- The term pyranose means a six-membered sugar ring (hemiacetal or acetal - see below)
- the term furanose means a five-membered ring
- These terms are often prefixed as in "glucopyranose" which means glucose cyclized to its six-membered ring form (see below)
4. D- and L-Sugars:
This is a naming convention. If using standard nomenclature numbering, the highest numbered stereogenic center ("chiral center" or "asymmetric center") has R configuration, the sugar is a D-sugar; if it has S-configuration, the sugar is an L-sugar.
5. Glucose Hemi-Acetal Formation:
The open form of D-glucose (and many other sugars) can cyclize to form hemiacetals. These can be depicted in various ways as shown below. Under acidic conditions the hemiacetal form of glucose can react with other alcohols to give acetals known as glycosides. These are widely distributed in nature.
Fischer Projection:
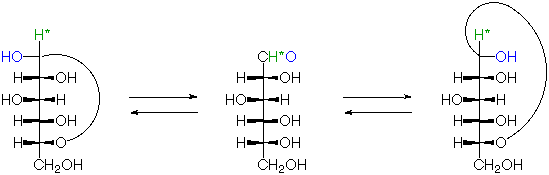
Same as Above in 3D View:
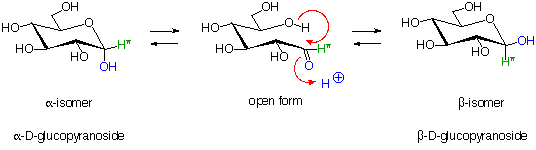
All of the above structures can be referred to as "D-glucose"
6. Glucose Acetal (Glycoside) Formation:
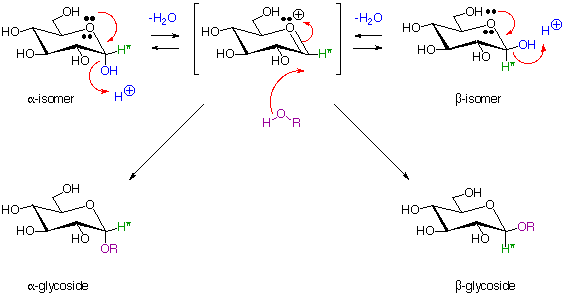
7. Examples of Disaccharides:
In the above structures, the anomeric carbons (acetal or hemiacetal) are indicated by coloured arrows. The full systematic names of the sugars are given below their common names.