Substitution & Elimination Reactions: SN2 , SN1 , E2 , & E1
SN2 Reaction
SN1 Reaction
E2 Reaction
E1 Reaction
Download pdf
- Nucleophilic Substitution Reactions (SN2 and SN1) replace a leaving group with a nucleophile (Nu: or Nu: - )
- Elimination Reactions (E2 and E1) generate a double bond by loss of " A+ " and " B: - "
- They may compete with each other
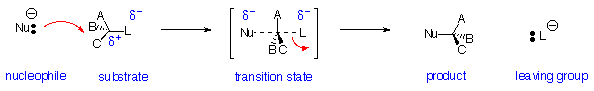
Nucleophilic Substitution Reactions - SN2 Reaction:
Reaction is:
- Stereospecific (Walden Inversion of configuration)
- Concerted - all bonds form and break at same time
- Bimolecular - rate depends on concentration of both nucleophile and substrate
- Substrate: Best if primary (one substituent on carbon bearing leaving group), works if secondary, fails if tertiary
- Nucleophile: Best if more reactive (i.e. more anionic or more basic)
- Leaving Group: Best if more stable (i.e. can support negative charge well):
- TsO- (very good) > I- > Br- > Cl- > F- (poor)
- RF , ROH , ROR , RNH2 are NEVER Substrates for SN2 reactions
- Leaving Groups on double-bonded carbons are never replaced by SN2 reactions
- Solvent: Polar Aprotic (i.e. no OH) is best: for example dimethylsulfoxide ( CH3SOCH3 ), dimethylformamide ( HCON(CH3)2 ), acetonitrile ( CH3CN ). Protic solvents (e.g. H2O or ROH) deactivate nucleophile by hydrogen bonding but can be used in some cases
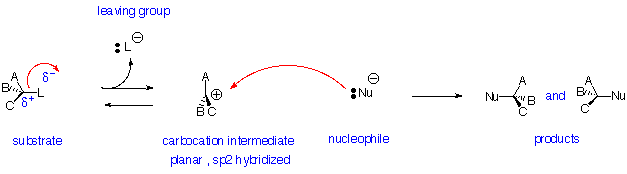
Nucleophilic Substitution Reactions - SN1 Reaction:
Reaction is:
- Non-stereospecific (attack by nucleophile occurs from both sides)
- Non-concerted - has carbocation intermediate
- Unimolecular - rate depends on concentration of only the substrate
Substrate: Best if tertiary or conjugated (benzylic or allylic) carbocation can be formed as leaving group departs, never primary
Nucleophile: Best if more reactive (i.e. more anionic or more basic)
Leaving Group: Same as SN2: best if more stable (i.e. can support negative charge well):
TsO- (very good) > I- > Br- > Cl- > F- (poor)
However, tertiary or allylic ROH or ROR' can be reactive under strongly acidic conditions to replace OH or OR
Solvent: Same as SN2: Polar Aprotic (i.e. no OH) is best: for example dimethylsulfoxide ( CH3SOCH3 ), dimethylformamide ( HCON(CH3)2 ), acetonitrile ( CH3CN ). Protic solvents (e.g. H2O or ROH) deactivate but can be used in some cases
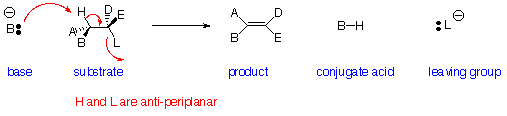
Elimination Reactions - E2 Reaction:
Reaction is:
- Stereospecific (Anti-periplanar geometry preferred, Syn-periplanar geometry possible)
- Concerted - all bonds form and break at same time
- Bimolecular - rate depends on concentration of both base and substrate
- Favoured by strong bases
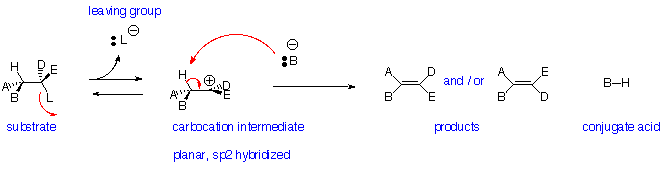
Elimination Reactions - E1 Reaction:
Reaction is:
- Non-stereospecific- follows Zaitsev (Saytseff) Rule
- Non-concerted - has carbocation intermediate - favoured for tertiary leaving groups
- Unimolecular - rate depends on concentration of only the substrate
- Does NOT occur with primary alkyl halides (leaving groups)
- Strong acid can promote loss of OH as H2O or OR as HOR if tertiary or conjugated carbocation can be formed