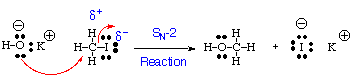Guide to Electron Bookkeeping & Arrow Pushing
Basic Rules
Basic Principles
Key Fundamental Examples
Other Useful Principles
Complex Example
Download pdf
Arrow pushing is a very useful electron bookkeeping device which allows us to:
1. rationalize and...
2. sometimes predict what will happen in a chemical reaction.
Basic Rules
- Atoms in stable molecules (final products) want to have inert gas configuration of electrons (2e- for H, 8e- in outer shell for 2nd row elements).
- Atoms in stable molecules want to avoid permanent generation of additional charge or separation of (+) and (-) charges, though this may occur temporarily during the reaction.
- Each bond represents a pair of electrons, and pushing additional electrons onto an atom which already has an inert gas configuration forces a bond cleavage.
- Unshared electrons resulting in negative charge generation are best stored on highly electronegative elements (e.g., halogen, oxygen) or in highly resonance stabilized systems (especially ones containing electronegative elements such as oxygen or nitrogen).
Basic Principles
Like charges repel, unlike charges attract. Atoms want to have the electronic configuration of inert gases. For example:
- 2 e- around H
- 8 e- in outer shell around C, N, O, F
Hence in molecules:
- Stable uncharged carbons will have 4 bonds (each bond is 2 e-) and no lone pairs of electrons.
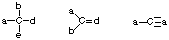
- Stable uncharged nitrogens will have 3 bonds and one lone pair of electrons.
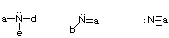
- Stable uncharged oxygens will have 2 bonds and 2 lone pairs of electrons.

- Stable uncharged halogens (F, Cl, Br, I) will have one bond and 3 lone pairs of electrons (remaining outer shell electrons in higher halogens are ignored in this course).
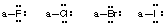
- Stable uncharged hydrogens will have one bond and no lone pairs of electrons (He electronic configuration).
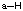
KEY Fundamental Examples:
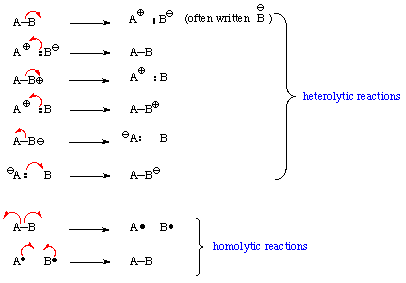
Electrons will tend to go from areas of high density (nucleophilic sites) to areas of insufficient density (electrophilic sites). Use a curved double headed arrow to move a pair of electrons (e.g., a bond) from where they are to where they are not. Use a single-headed arrow to move one electron:
A Simple Practical Example:
The reaction above could in principle (though not in practice) also be drawn in the reverse direction to form KOH and methyl iodide from methanol and KI. The convention is unfortunately not a substitute for thinking. Knowledge of relative electronegativity and stability of anions and cations is essential. However, it is easy to see that iodine is happier as iodide than oxygen is as hydroxide. You can certainly eat salt containing small amounts of sodium or potassium iodide; this would be inadvisable for salt containing KOH or NaOH.
Other Useful Principles
- Push the electrons from the most nucleophilic center toward the electrophilic center with an eye to generating the bonds required in the product.
- Protons (H+) are very mobile if they are attached to an electronegative element.
- Avoid putting two like charges on the same atom or adjacent atoms.
- The desired overall effect is to redistribute electron density more evenly; this may require a momentary unfavorable fluctuation (e.g., further separation or generation of charge).
- If you push electrons onto an atom having a full outer shell, a bond to that atom must break.
- Remember :
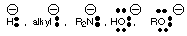
(where R = alkyl) are terrible leaving groups. Hydride will only be removed from a molecule by an oxidizing agent. Hydroxide, amide anion, and alkoxide will often leave easily if they are protonated first. The carbanion will leave only if the negative charge ion carbon is resonance stabilized by an electronegative atom or group.
- The nucleophilicity of lone pairs on hetero atoms decreases (other things being equal) in the order:
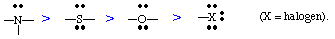
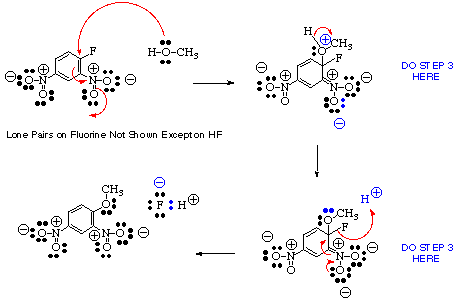
Complex Example:
- Draw in all of the lone pairs, formal charges and carbon-hydrogen bonds near the sites where reaction may take place (often the whole molecule must be considered).
- For example:
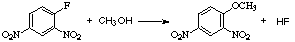
- Becomes:
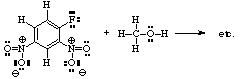
- Formal charge can always be calculated by: e.g., for nitrogen above:
+ number of protons in nucleus of atom. +7 - number of electrons in inner shell -2 - number of electrons unshared in outer shell. -0 - (1/2) x number of electrons shared in outer shell. -4 Formal Charge on Atom +1
- For example:
- If the product is given, decide which atoms in the products are derived from which atoms in the starting materials, and possibly redraw the starting materials to resemble the products
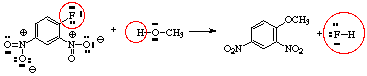
- (a) Identify the most nucleophilic center(s) in the starting materials (often an unshared pair of electrons on a hetero atom or sometimes a multiple bond).
(b) Identify the most electrophilic center(s) in the starting materials. This will frequently be a proton (H+), a multiply-bonded electronegative element, or a carbon bearing on electronegative group. Look for sites where electrons can be stabilized by resonance (e.g., from aromatic systems or enol(ate) systems if possible.)
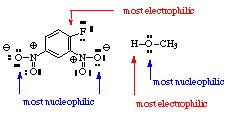
- Decide whether the reaction is likely to be homolytic (radical) bond cleavage, electrocyclic, or heterolytic (polar) bond cleavage. The former often involve heat or light, the latter usually involves acid or base
- In the example, the reaction is likely to be heterolytic since the O-H bond of CH3OH is easily broken and oxygen is much more electronegative than hydrogen.
- Draw out completely the structures of the intermediates showing all formal charges before proceeding to the next step in a multistep process. Go back to step 3 to determine the next step.