Chem 164 - Additional Graphics
Acetylene (ethyne): C2H2
- the carbons are sp hybridized
- the arrangement at each C is linear
- the bond angles are 180 degrees
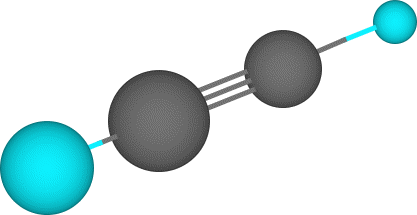
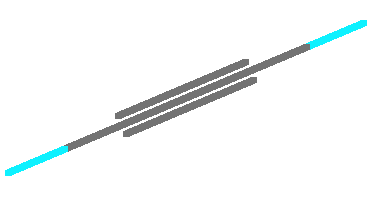
The following images show the molecule in various renderings. Carbon is grey, the hydrogens are blue.
Ball & Stick Display |
Wire Frame Display |
Space Filling Display |
 |
 |
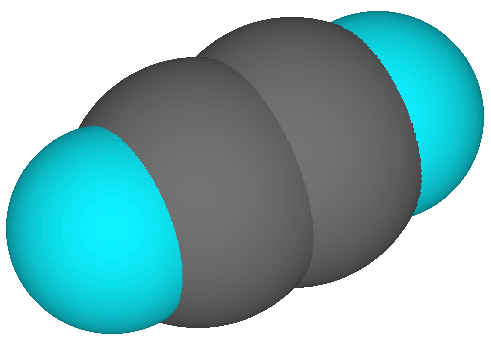 |
3D Ball & Stick Display (view with 3D glasses) |
 |
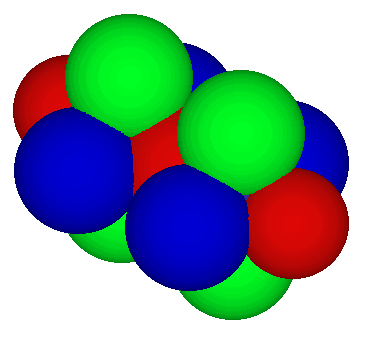
Orbitals on carbon:
- Two green p atomic orbitals are shown, one on each carbon ready to form a pi molecular orbital
- Two blue p atomic orbitals are shown, one on each carbon ready to form a second pi molecular orbital at right angles to the first
- a red sigma molecular orbital formed by overlap of two sp atomic orbitals connects the carbons which are not visible in the drawing below
- two additional red sp atomic orbitals are shown at each end and are ready to form sigma molecular orbitals with hydrogen s atomic orbitals