Reactions of Carboxylic Acids and Derivatives
Common Names
Addition to Carbonyl
Addition at Alpha Carbon
Download pdf
Important Common Names of Carboxylic Acids Include:
Systematic Name
Common Name
Structure
methanoic acid
formic acid
HCO2H
ethanoic acid
acetic acid
CH3CO2H
propanoic acid
propionic acid
CH3CH2CO2H
butanoic acid
butyric acid
CH3CH2CH2CO2H
pentanoic acid
valeric acid
CH3CH2CH2CH2CO2H
benzoic acid
benzoic acid
PhCO2H
ethanedioic acid
oxalic acid
HO2C-CO2H
propanedioic acid
malonic acid
HO2CCH2CO2H
butanedioic acid
succinic acid
HO2CCH2CH2CO2H
pentanedioic acid
glutaric acid
HO2CCH2CH2CH2CO2H
hexanedioic acid
adipic acid
HO2CCH2CH2CH2CH2CO2H
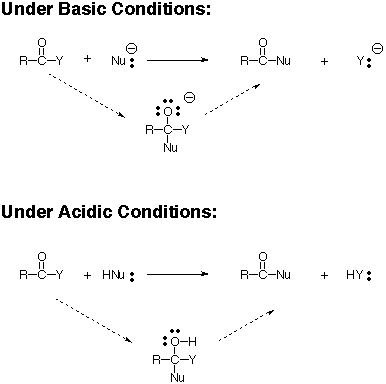
Reactions of carboxylic acids and their derivatives (acyl halides, anhydrides, esters, amides) resemble those of ketones and aldehydes, but replacement (substitution) of an electronegative group on the carbonyl is the common extra feature. Most reactions follow a pattern:
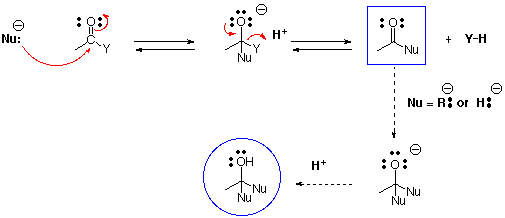
1. Addition to carbonyl by strong (irreversible) or weak (reversible) nucleophiles with expulsion of an electronegative group.The strong nucleophiles (hydride and alkyl anion) usually add a second time to the aldehyde or ketone that has been generated by the first addition2. Reaction at alpha carbon with an electrophile via enolate
1. Addition to Carbonyl (Compare to ketone reaction)
In the first step, nucleophiles generally attack the carbonyl of carboxylic acid derivatives (e.g. acid chlorides, anhydrides, esters, amides) in the same way as with ketones and aldehydes. However, the presence of an electron withdrawing atom directly attached to the former carbonyl carbon allows its expulsion to generate a new carbonyl derivative in which the nucleophile has replaced the heteroatom group (Y). In the case of interconversions of carboxylic acid derivatives with heteroatom (weak) nucleophiles, this gives the final product in the box.
If the nucleophile is very strong, such as R: - (alkyl anion from RLi or RMgX) or H: - (hydride anion from LiAlH4 or NaBH4), there is no H+ present (the positive ion is a metal) and the product in the box is a ketone or aldehyde which will react with a second mole of nucleophile (see ketone reactions) to ultimately generate an alcohol (in the circle). Amides are an exception and give amines with LiAlH4 or NaBH4. In order to decide whether a reaction with a heteroatom nucleophile will work, the scheme shown below is useful.
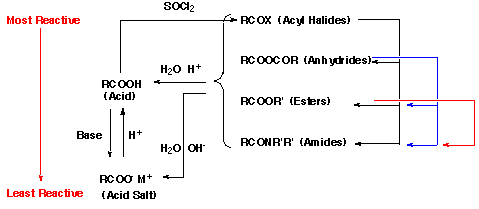
In general, carboxylic acid derivatives that are more reactive can be spontaneously converted into less reactive ones by treatment with the appropriate nucleophilic species. The exceptions are carboxylic acids which will generally react with alcohols to form esters only under acidic conditions because under basic conditions the formation of unreactive salts is extremely rapid. The general reaction for formation of carboxylic acid derivatives is:
The above schemes can be used to determine possible routes to convert one carboxylic acid derivative into another. For example, to convert acetamide [CH3CONH2] into N,N-dimethylacetamide [CH3CON(CH3)2] , it is necessary to hydrolyze first to the carboxylic acid with water and acid (or to the carboxylate salt with water and base (e.g. KOH) ), next convert to the acid chloride with thionyl chloride, and then treat with dimethylamine [ HN(CH3)2 ]. In contrast, acetic anhydride [CH3COOCOCH3] or ethyl acetate [CH3COOCH2CH2] can be converted to N,N-dimethylacetamide [CH3CON(CH3)2] directly by treatment with dimethylamine [ HN(CH3)2 ].
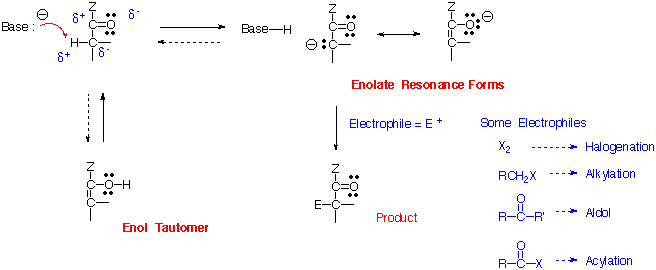
2. Reaction at Alpha Carbon (Compare to ketone reaction)
The general features of this type of reaction are analogous to the corresponding reactions of aldehydes and ketones. Of course the products will now be carboxylic acid derivatives. This type of reaction is particularly useful with esters. The base must be chosen carefully such that it does not act as a nucleophile and attack the carbonyl in a "Type 1" reaction. For esters, LDA (lithium diisopropylamide) at low temperature is especially useful as a base.