Your choice (acidic) is 
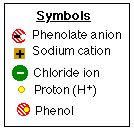
- Note that there are extra protons (H+) in the
solution.
- Note also that sodium chloride, (NaCl), a bi-product of the
reaction, is dissolved in the aqueous solution while the phenol*
has oiled out* of the solution.
Now you need to collect the crude phenol that has not
crystallized, but oiled out.
 What technique will you use?
What technique will you use?
Organic Web Chem University of Alberta
![]()
What technique will you use?