Your choice (acidic) is 
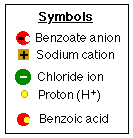
- Note that there are extra
protons (H+) in the solution.
- Note also that sodium
chloride, (NaCl), a bi-product of the reaction, is
dissolved in the aqueous solution while the benzoic acid* has precipitated* out of the solution.
Now you need to collect the
benzoic acid. What technique will you use?
Organic Web Chem University of Alberta
![]()