Isolation of
benzoic acid
OK, Let's isolate benzoic acid* from the solution which contains it.
- A basic aqueous solution
contains the salt* of benzoic acid, sodium benzoate*.
- You want to change sodium
benzoate (salt of an organic acid) to the water-insoluble
benzoic acid (organic acid) by converting the salt to a
protonated, non-ionic* form.
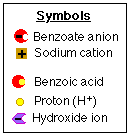
Which solution will you add?
Organic Web Chem University of Alberta