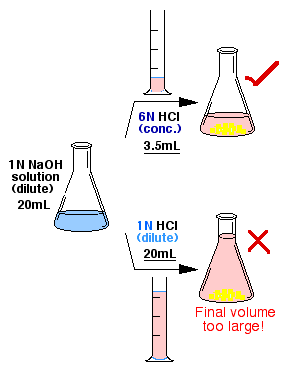
- The recovery of organic acids requires
acidification with concentrated HCl solution. Recall that
in the extraction step for the separation of an organic acid
either dilute NaHCO3 or NaOH was used. Concentrated
HCl will now help minimize the volume of the final aqueous solution.
- Acidification must be done carefully,
because the acid-base neutralization reaction is quite exothermic.
- Check the pH of the solution to make sure
that it is acidic. (~pH 3)
-
-
-
-
-
-
Organic Web Chem University of Alberta