Let's see another sample problem.
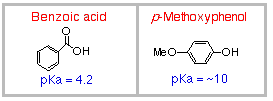
This time you have a mixture ofphenol
and benzoic acid, dissolved
in dichloromethane.
How do you separate these two compounds?
Answer:
To extract benzoic acid (a carboxylic acid), usually you
may use either5% NaOH or sat. NaHCO3
HOWEVER, you cannot use NaOH solution this time.
It extracts both phenol
and benzoic acid!
Do you still want to try NaOH solution? Click this button.
 Reset
Reset
Use NaHCO3 solution! (This is the right
choice.)
 Reset
Reset
Did you see that you separated the two compounds using NaCO3
aqueous solution?
Let's continue then...
Reset