Research –
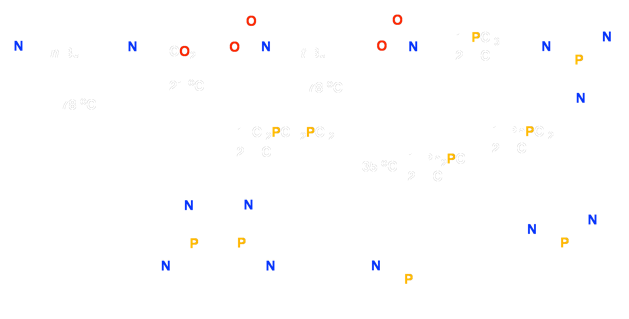
We have recently synthesized a new series of “hybrid” ortho-phosphinoaniline ligands possessing both soft phosphine and hard amine donors (Figure 1). When coordinated to late transition metals (specifically Rh and Ru) these ligands can give rise to hemilability in which the soft phosphorus terminus binds more strongly to the soft metal center, effectively maintaining the integrity of the complex, while the more weakly binding nitrogen donor(s) may be labile to displacement by substrate molecules, providing a degree of incipient coordinative unsaturation.
Hemilabile P/N Ligands








Virtual coordinative Unsaturation
For more information, see Lindsay’s work
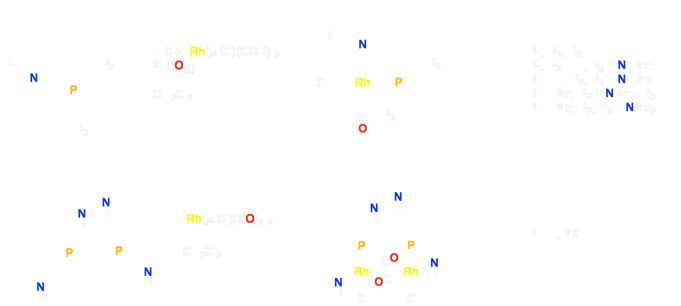
The fluxionality inherent within such hemilabile complexes can be studied by variable temperature, multinuclear NMR spectroscopy and this method has allowed us to determine that the extent of methyl substitution at the amine substantially alters its lability at the metal. Mono- and binuclear complexes bearing amines with either one or two methyl substituents (Figure 2) have been analyzed by X-ray crystallography. Solid state structural analysis provides a rationale for the variable degrees of hemilability based on steric congestion at the metal center(s).
In the case of the ruthenium complexes, we have found that by changing the number of available donor on the ligands, the degree of methylation at the amines and oxidation states of the metals, we can achieve a variety of interesting coordination modes at the metal center. These complexes are also expected to be catalyst precursors for hydrogenation and hydroformylation reactions and reactivity studies are underway. The effects of bimetallic cooperativity in such reactions can be inferred by comparing activities and selectivities of the bimetallic catalysts with the related monometallic analogues.
Figure 1
Figure 2