Mohammad R. Momeni, Eric Rivard, and Alex Brown
Department of Chemistry, University of Alberta, Edmonton, Alberta T6G 2G2, Canada
Abstract
Mohammad R. Momeni, Eric Rivard, and Alex Brown
Department of Chemistry, University of Alberta, Edmonton, Alberta T6G 2G2, Canada

Abstract
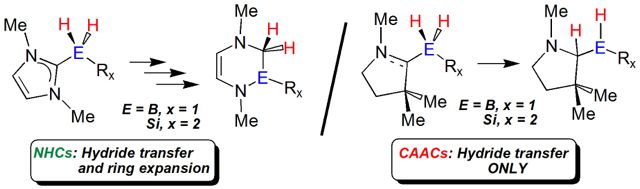
The stability of a variety of borane (BH3 and BH2NHMe) and silane (SiHnPh4-n, n = 0-4) adducts with diamino (NHC) and aminoalkyl (CAAC) carbenes has been carefully examined using M06-2X/cc-pVDZ computations, including natural bond orbital and atoms-in-molecules analyses. Moreover a potential mechanism for the hydride-mediated ring expansion of the carbene donors is reported. While the NHC adducts can undergo thermally induced ring-expansion chemistry, the CAAC adducts show increased stability due to a large energetic barrier for the insertion of boron (or silicon) atoms into the CAAC heterocycle. A series of substituted NHCs were investigated to further explore the roles of both electronic and steric effects on adduct stabilities and on their propensities for undergoing ring-expansion transformations.
Return to Dr. Alex Brown's Publications
This page maintained by alex.brown@ualberta.ca of the Department of Chemistry, University of Alberta
Last updated November 13, 2013.