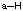Basic Principles
Like charges repel, unlike charges attract. Atoms want to have the electronic configuration of inert gases. For example:
- 2 e- around H
- 8 e- in outer shell around C, N, O, F
Hence in molecules:
- Stable uncharged carbons will have 4 bonds (each bond is 2 e-) and no lone pairs of electrons.
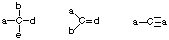
- Stable uncharged nitrogens will have 3 bonds and one lone pair of electrons.
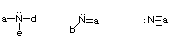
- Stable uncharged oxygens will have 2 bonds and 2 lone pairs of electrons.

- Stable uncharged halogens (F, Cl, Br, I) will have one bond and 3 lone pairs of electrons (remaining outer shell electrons in higher halogens are ignored in this course).
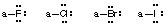
- Stable uncharged hydrogens will have one bond and no lone pairs of electrons (He electronic configuration).